Elements different types of atom Elements are the






- Slides: 10

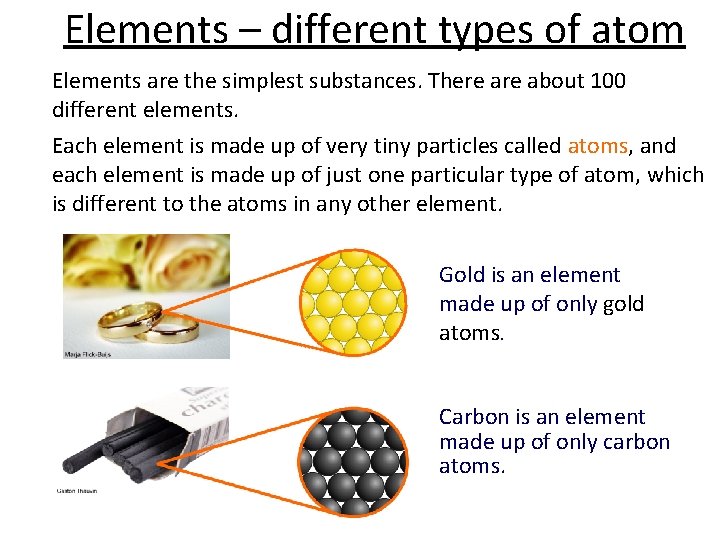
Elements – different types of atom Elements are the simplest substances. There about 100 different elements. Each element is made up of very tiny particles called atoms, and each element is made up of just one particular type of atom, which is different to the atoms in any other element. Gold is an element made up of only gold atoms. Carbon is an element made up of only carbon atoms.

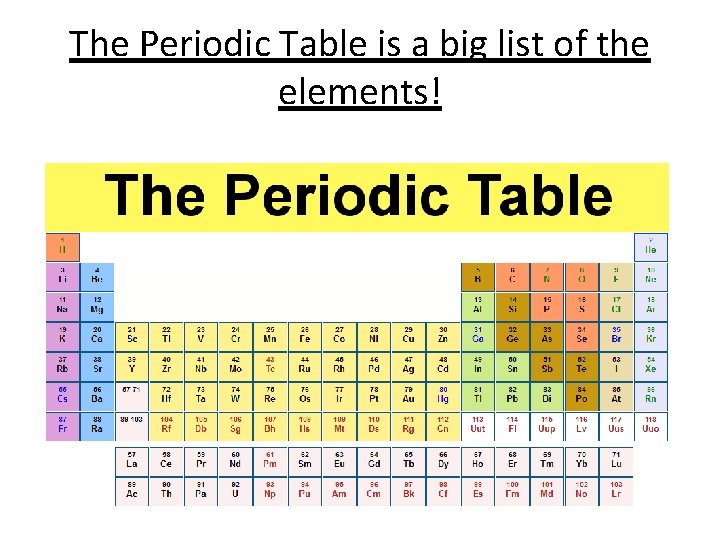
The Periodic Table is a big list of the elements!

The beginnings of the periodic table • By the 1860’s 63 elements were known. Chemists were keen to make a table that organised the elements in a helpful way, but this proved difficult. • In one table of elements, every 8 th elements had similar properties. Unfortunately this worked only as far as calcium so it was a success.

Dmitri Mendeleev (1834 -1907) • Then in 1869, the Russian chemist Dmitri Mendeleev (1834 -1907) was busy writing the second volume of his chemistry textbook. • He was having troubling deciding which elements to write about next. Mendeleev’s solution was to construct a table.

The Periodic Table • Mendeleev’s table is now called the periodic table. To make his original table, Mendeleev used the latest measurements of atomic masses (called ‘weights’ then) available. • He also carefully considered the properties of the different elements.

Atomic Mass • Mendeleev arranged the elements in order of increasing atomic mass. Other chemists had tried this before, but Mendeleev sometimes broke this rule. • He did so that elements was similar properties lined up in his able. For example iodine should come before tellurium according to its atomic mass. • Mendeleev swapped the positions of these two elements that they lined up with the elements with similar properties.

Leaving Gaps • Mendeleev put elements with similar properties into horizontal rows in his first table. • However with similar properties organised into vertical columns, just as in the modern periodic table. Unlike other chemicals, Mendeleev thought that there must be elements still to discover, so he left gaps for them.


Making Predictions • Mendeleev used the gaps in his table to make predictions about the properties of undiscovered elements. One of these predictions was for an elements that he called eka-aluminium. • When gallium was discovered shortly afterword in 1875, its properties closely fitted those he had predicted for ekaaluminium.

Key Points about the Modern Periodic Table • Elements are arranged in order of increasing atomic number (the number of protons) rather than in order of atomic mass as Mendeleev did. • Horizontal rows are called periods • Vertical Columns are called groups • Each group contains elements with similar properties, the main groups are numbered 1 to 7 left to right. The group on the far right is group 0.