ELECTRON TRANSPORT CHAIN NADH and FADH 2 transfer



- Slides: 8

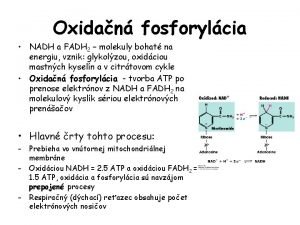
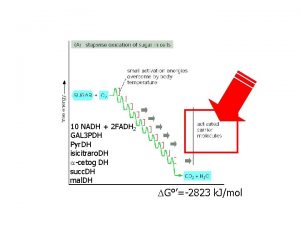
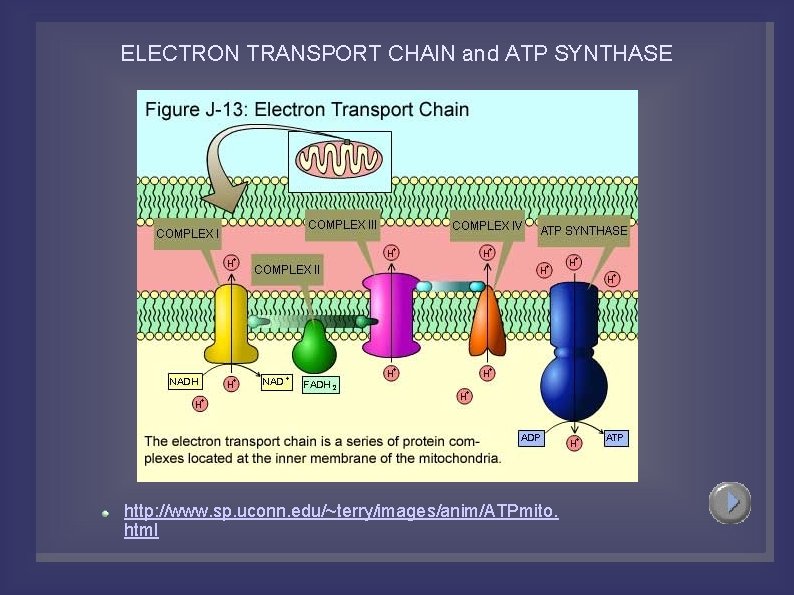
ELECTRON TRANSPORT CHAIN NADH and FADH 2, transfer their electrons to a series of compounds (mostly proteins), which are associated with the inner mitochondrial membrane. The protein/compounds are arranged in order of increasing electronegativity (each successive compound wants the electrons more than the one before it). The compounds (in order): NADH dehydrogenase, ubiquinone (Q), the cytochrome b-c 1 complex, cytochrome c, cytochrome oxidase complex. Each compound is reduced by gaining two electrons from the one before it and oxidized by donating its two elecrtons to the one after it. As the electrons are passed they become more stable and therefore generate free energy. This free energy is used to pump protons into the intermembrane space from the matrix (active transport). There are 3 proton pumps. Oxygen is the final electron acceptor and it joins with two protons in the matrix to form water.


ELECTRON TRANSPORT CHAIN Steps: NADH, from pyruvate oxidation and the Krebs Cycle, gives up its two electrons to NADH dehydrogenase, The mobile carriers Q and cytochrome c shuttle electrons from one protein complex to the next until they reach the cytochrome oxidase complex (final acceptor). Each protein complex (3) also acts as a proton pump, using the free energy released to move protons from the matrix to the intermembrane space. At the cytochrome oxidase complex, cytochrome oxidase, catalyzes the reaction between the electrons, protons and oxygen to form water. (2 H+ + 1/2 O 2 --> H 2 O) This process is highly exergonic (giving up free energy of 222 k. J/mol). The chemical potential energy of the electron position is converted to the electrochemical potential energy of a proton gradient that forms across the inner mitochondrial membrane. High concentration in the intermembrane space, low concentration in the matrix. The proton gradient will be used to produced ATP.


ELECTRON TRANSPORT CHAIN http: //www. sp. uconn. edu/~terry/images/anim/ETS. html FADH 2 skips the first protein compound (starts at Q). This means that NADH oxidation pumps three protons into the intermembrane space, while FADH 2 oxidation pumps only two protons. Three ATP are formed from NADH while two ATP are formed from FADH 2. . Once NADH and FADH 2 are oxidized they pick up more H+ in glycolysis, pyruvate oxidation, or the Kreb's cycle (recycling of electron carriers). Important to note that the NADH formed in glycolysis in the cytoplasm cannot get to the matrix to take part in the ETC. However, it passes into the mitochondrial matrix through the glycerol-phosphate shuttle, where its electrons are passed to FADH 2, therefore another FADH 2 is essentially created in glycolysis, and from it another 2 ATP are formed. There is another way that NADH can pass its electrons to another NAD+ instead of FAD. . . it is the aspartate shuttle, but we will ignore this one. There are many copies of the ETC along the cristae, therefore lots of ATP can be produced.

CHEMIOSMOSIS and OXIDATIVE ATP SYNTHESIS (Phosphorylation) There is an electrochemical gradient across the intermembrane space. (More protons outside than in the matrix). Two parts: difference in charge and a difference in concentration. The inner membrane is impermeable to protons. The protons are forced through special proton channels that are coupled with ATP synthase (ATPase). The electrochemical gradient produces a proton-motive force (PMF) that moves the protons through this ATPase complex. Each time a proton comes through the ATPase complex, the free energy of the electrochemical gradient is reduced and this energy is used to create ATP from ADP + P in the matrix.

ELECTRON TRANSPORT CHAIN and ATP SYNTHASE http: //www. sp. uconn. edu/~terry/images/anim/ATPmito. html

CHEMIOSMOSIS and OXIDATIVE ATP SYNTHESIS (Phosphorylation) Peter Mitchell found all this out in 1961 and coined the term chemiosmosis because the energy that drives ATP production comes from the osmosis of protons. It took a long time for his theory to be accepted. He finally got his Nobel Prize in 1978. The continual production of ATP is dependent on the maintenance of a proton reservoir in the intermembrane space. This depends on the continued movement of electrons and that depends on the availability of oxygen. Therefore we need oxygen to prevent the ETC from being clogged up and we need food to provide the glucose that provides electrons for the ETC.