Early Phase Renal Denervation Trial Design in Stage



- Slides: 10

Early Phase Renal Denervation Trial Design in Stage 2 Hypertension William B. White, MD Professor and Division Chief Hypertension and Clinical Pharmacology Calhoun Cardiology Center University of Connecticut School of Medicine Farmington, Connecticut

Faculty Disclosure • Research Funding – National Institutes of Health • Consulting activities during past 12 months that are related or relevant to this discussion – Safety consultant – Takeda Development Center, Inc • Officer of the American Society of Hypertension (Immediate Past President)

Considerations for a Phase I-II RDN Trial • There are concerns regarding differences between controlled and uncontrolled RDN trials • Prior to exposure of > 600 patients in a phase III RDN trial, there really must be evidence of the efficacy of the device • A smaller trial in untreated stage II patients with hypertension could be performed to assess device efficacy • Results of this type of study could be utilized by regulators as part of the registration package

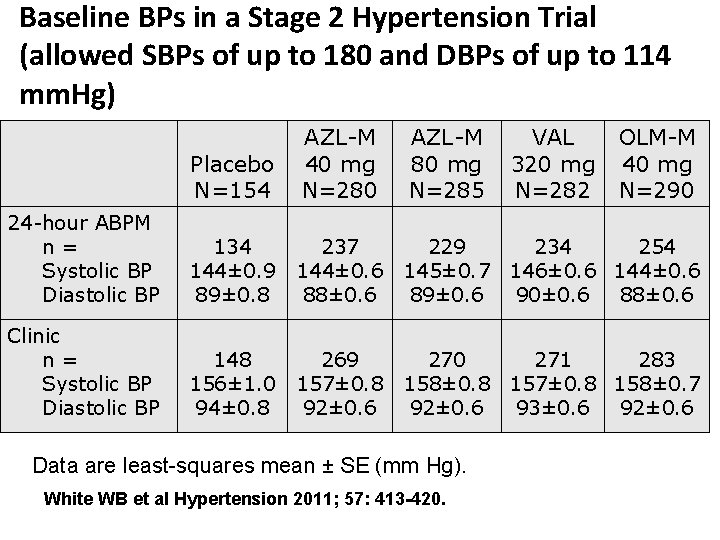
Baseline BPs in a Stage 2 Hypertension Trial (allowed SBPs of up to 180 and DBPs of up to 114 mm. Hg) Placebo N=154 AZL-M 40 mg N=280 AZL-M 80 mg N=285 24 -hour ABPM n= Systolic BP Diastolic BP 134 144± 0. 9 89± 0. 8 237 229 234 254 144± 0. 6 145± 0. 7 146± 0. 6 144± 0. 6 88± 0. 6 89± 0. 6 90± 0. 6 88± 0. 6 Clinic n= Systolic BP Diastolic BP 148 156± 1. 0 94± 0. 8 269 270 271 283 157± 0. 8 158± 0. 8 157± 0. 8 158± 0. 7 92± 0. 6 93± 0. 6 92± 0. 6 Data are least-squares mean ± SE (mm Hg). White WB et al Hypertension 2011; 57: 413 -420. VAL 320 mg N=282 OLM-M 40 mg N=290

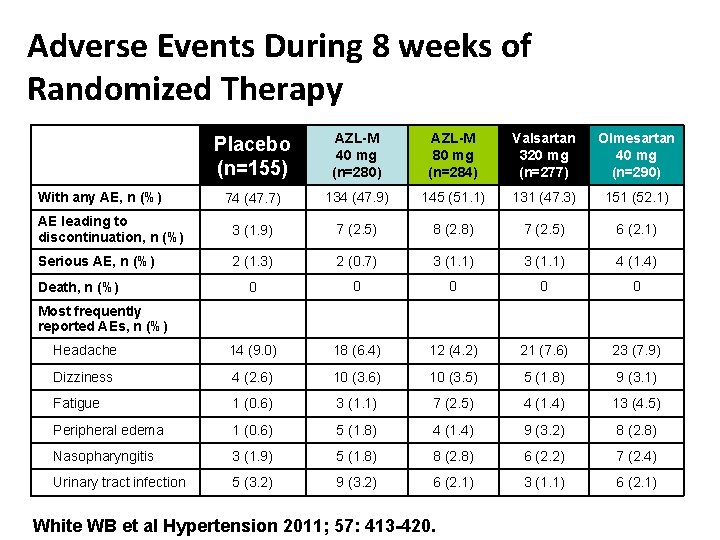
Adverse Events During 8 weeks of Randomized Therapy Placebo (n=155) AZL-M 40 mg (n=280) AZL-M 80 mg (n=284) Valsartan 320 mg (n=277) Olmesartan 40 mg (n=290) 74 (47. 7) 134 (47. 9) 145 (51. 1) 131 (47. 3) 151 (52. 1) AE leading to discontinuation, n (%) 3 (1. 9) 7 (2. 5) 8 (2. 8) 7 (2. 5) 6 (2. 1) Serious AE, n (%) 2 (1. 3) 2 (0. 7) 3 (1. 1) 4 (1. 4) 0 0 0 Headache 14 (9. 0) 18 (6. 4) 12 (4. 2) 21 (7. 6) 23 (7. 9) Dizziness 4 (2. 6) 10 (3. 5) 5 (1. 8) 9 (3. 1) Fatigue 1 (0. 6) 3 (1. 1) 7 (2. 5) 4 (1. 4) 13 (4. 5) Peripheral edema 1 (0. 6) 5 (1. 8) 4 (1. 4) 9 (3. 2) 8 (2. 8) Nasopharyngitis 3 (1. 9) 5 (1. 8) 8 (2. 8) 6 (2. 2) 7 (2. 4) Urinary tract infection 5 (3. 2) 9 (3. 2) 6 (2. 1) 3 (1. 1) 6 (2. 1) With any AE, n (%) Death, n (%) Most frequently reported AEs, n (%) White WB et al Hypertension 2011; 57: 413 -420.

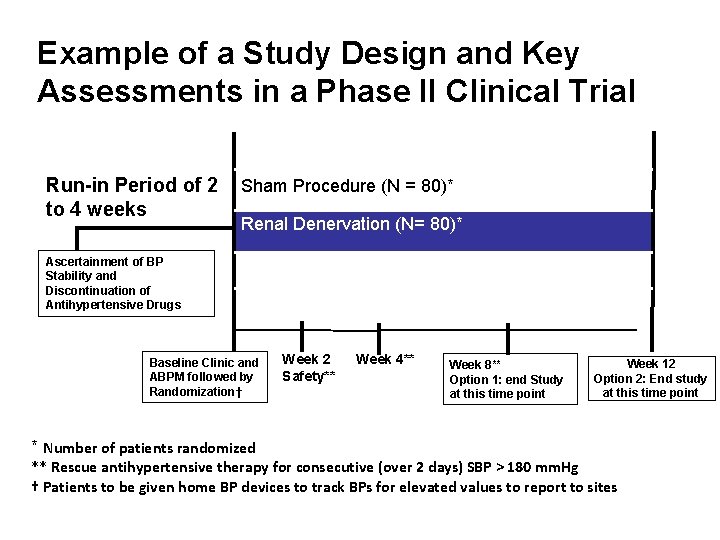
Example of a Study Design and Key Assessments in a Phase II Clinical Trial Run-in Period of 2 to 4 weeks Sham Procedure (N = 80)* Renal Denervation (N= 80)* Ascertainment of BP Screening Stability and (Day. Discontinuation -28 to -21)of Antihypertensive Drugs Baseline Clinic and ABPM followed by Randomization† * Number of patients randomized Week 2 Safety** Week 4** Week 8** Option 1: end Study at this time point Week 12 Option 2: End study at this time point ** Rescue antihypertensive therapy for consecutive (over 2 days) SBP > 180 mm. Hg † Patients to be given home BP devices to track BPs for elevated values to report to sites

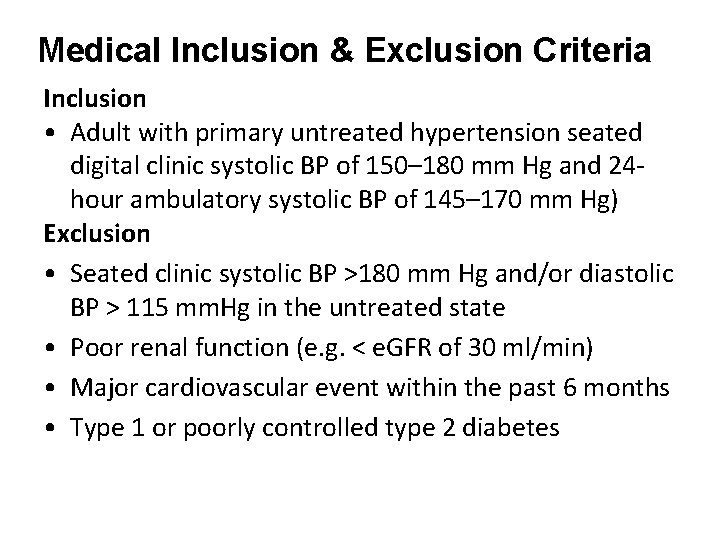
Medical Inclusion & Exclusion Criteria Inclusion • Adult with primary untreated hypertension seated digital clinic systolic BP of 150– 180 mm Hg and 24 hour ambulatory systolic BP of 145– 170 mm Hg) Exclusion • Seated clinic systolic BP >180 mm Hg and/or diastolic BP > 115 mm. Hg in the untreated state • Poor renal function (e. g. < e. GFR of 30 ml/min) • Major cardiovascular event within the past 6 months • Type 1 or poorly controlled type 2 diabetes

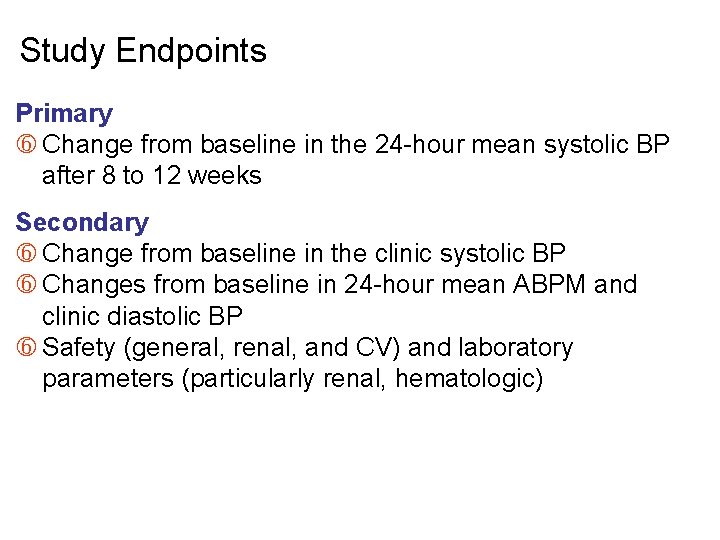
Study Endpoints Primary Change from baseline in the 24 -hour mean systolic BP after 8 to 12 weeks Secondary Change from baseline in the clinic systolic BP Changes from baseline in 24 -hour mean ABPM and clinic diastolic BP Safety (general, renal, and CV) and laboratory parameters (particularly renal, hematologic)

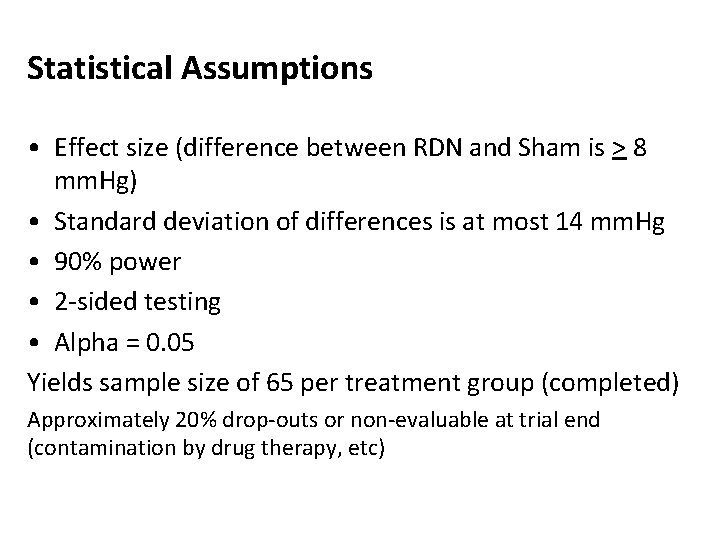
Statistical Assumptions • Effect size (difference between RDN and Sham is > 8 mm. Hg) • Standard deviation of differences is at most 14 mm. Hg • 90% power • 2 -sided testing • Alpha = 0. 05 Yields sample size of 65 per treatment group (completed) Approximately 20% drop-outs or non-evaluable at trial end (contamination by drug therapy, etc)

Concerns that have brought up regarding an early Phase RDN Trial • Drug washout is safe in this population but may confuse IRBs • An 8 or 12 week observation period could be too short • Ethical concerns of the procedure in patients off antihypertensive therapy – which level of data from prior research should support procedural safety? • If only drug-intolerant or non-adherent patients are selected, recruitment will take considerably longer and create generalizability concerns • How could results of this type of study be utilized by regulators as part of the registration package?