Dominick J Angiolillo MD Ph D Consulting Fees












![Clopiodgrel FDA Box Warnings The Code of Federal Regulations (21 CFR 201. 57 [e]) Clopiodgrel FDA Box Warnings The Code of Federal Regulations (21 CFR 201. 57 [e])](https://slidetodoc.com/presentation_image_h/b9e2cd48f7b74a7315992015daeecca0/image-20.jpg)




- Slides: 25

Dominick J. Angiolillo, MD, Ph. D § Consulting Fees – sanofi-aventis – Bristol-Myers Squibb – Eli Lilly and Company – Daiichi Sankyo

§ Honoraria – Bristol-Myers Squibb – sanofi-aventis – Eli Lilly and Company – Daiichi Sankyo I intend to reference off label or unapproved uses of drugs or devices in my presentation. I intend to discuss ticagrelor, elinogrel, cangrelor, TRA

Clopidogrel - PPI Interaction: The Interaction Is Real and the Warning Is Justified! Dominick J. Angiolillo, MD, Ph. D, FACC, FESC, FSCAI Director of Cardiovascular Research Associate Professor of Medicine University of Florida College of Medicine - Jacksonville

Clopidogrel – 2 C 19 specific PPI Interaction: The Interaction Is Real and the Warning Is Justified! Dominick J. Angiolillo, MD, Ph. D, FACC, FESC, FSCAI Director of Cardiovascular Research Associate Professor of Medicine University of Florida College of Medicine - Jacksonville

EFFECT ASPIRIN + CLOPIDOGREL prevention of cardiac damage SIDE EFFECT gastric bleeding PROTON EFFECT PUMP prevention of gastric bleeding INHIBITORS (PPIs)

2009 Updated Labeling for Clopidogrel–PPI Interaction • FDA-required label changes: 2 – Warning: “Co-administration of Plavix with omeprazole, a proton pump inhibitor that is an inhibitor of CYP 2 C 19, reduces the pharmacological activity of Plavix if given concomitantly or if given 12 hours apart” – Drug-Drug Interactions: “Avoid concomitant use of drugs that inhibit CYP 2 C 19, including omeprazole, esomeprazole, cimetidine, fluconazole, ketoconazole, voriconazole, etravirine, felbamate, fluoxetine, fluvoxamine, and ticlopidine” – Based on PK/PD studies showing concomitant omeprazole reduced clopidogrel active metabolite and effect on platelets 1 • Did not include COGENT study data 2 • EMEA warning extends to discourage concomitant use of all PPIs 3 – Concomitant use of drugs that inhibit CYP 2 C 19 discouraged; concomitant use of any PPI “should be avoided unless absolutely necessary” 4 EMEA=European Medicines Agency; FDA=Food and Drug Administration; PD=pharmacodynamic; PK=pharmacokinetic. 1 Food and Drug Administration. http: //www. fda. gov/Drugs/Drug. Safety/Postmarket. Drug. Safety. Informationfor. Patientsand. Providers/ Drug. Safety Informationfor. Heathcare. Professionals/ucm 190787. htm. Published November 17, 2009. Accessed January 22, 2010. 2 Plavix [package insert]. Bridgewater, NJ: Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; 2009. 3 Wathion N. http: //www. emea. europa. eu/humandocs/PDFs/ EPAR/Plavix/32895609 en. pdf. Published May 29, 2009. Accessed January 22, 2010. 4 Plavix [summary of product characteristics]. Paris, France: Sanofi Pharma Bristol-Myers Squibb SNC; 2009.

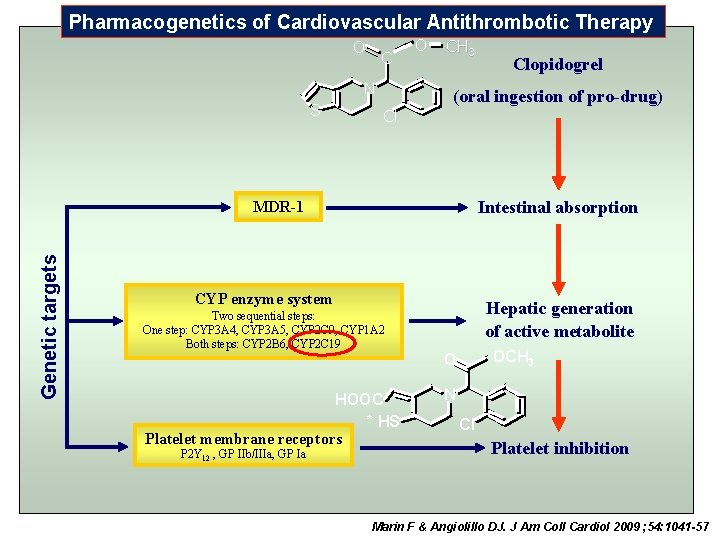
Pharmacogenetics of Cardiovascular Antithrombotic Therapy O C N S O CH 3 (oral ingestion of pro-drug) Cl Intestinal absorption Genetic targets MDR-1 CYP enzyme system Two sequential steps: One step: CYP 3 A 4, CYP 3 A 5, CYP 2 C 9, CYP 1 A 2 Both steps: CYP 2 B 6, CYP 2 C 19 HOOC * HS Platelet membrane receptors P 2 Y 12 , GP IIb/IIIa, GP Ia Clopidogrel Hepatic generation of active metabolite OCH 3 O N Cl Platelet inhibition Marin F & Angiolillo DJ. J Am Coll Cardiol 2009 ; 54: 1041 -57

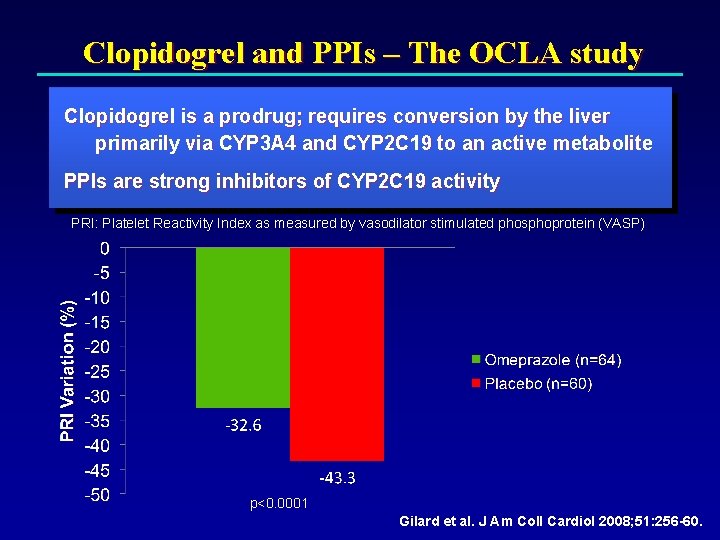
Clopidogrel and PPIs – The OCLA study Clopidogrel is a prodrug; requires conversion by the liver primarily via CYP 3 A 4 and CYP 2 C 19 to an active metabolite PPIs are strong inhibitors of CYP 2 C 19 activity PRI: Platelet Reactivity Index as measured by vasodilator stimulated phosphoprotein (VASP) p<0. 0001 Gilard et al. J Am Coll Cardiol 2008; 51: 256 -60.

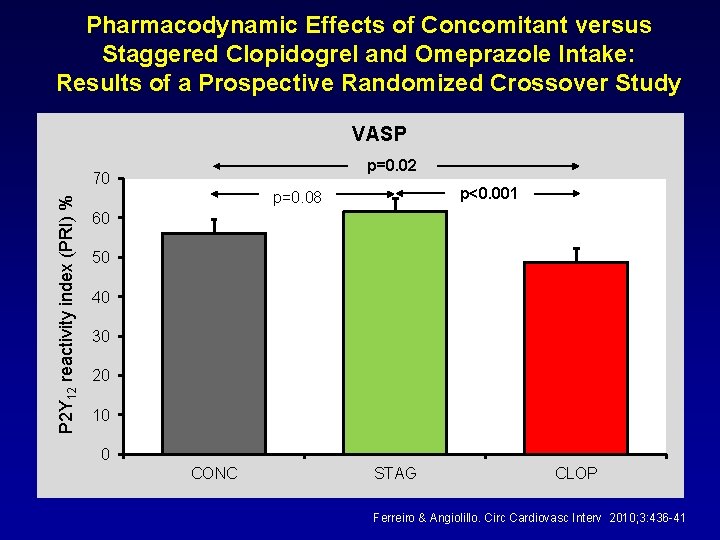
Pharmacodynamic Effects of Concomitant versus Staggered Clopidogrel and Omeprazole Intake: Results of a Prospective Randomized Crossover Study VASP p=0. 02 P 2 Y 12 reactivity index (PRI) % 70 p<0. 001 p=0. 08 60 50 40 30 20 10 0 CONC STAG CLOP Ferreiro & Angiolillo. Circ Cardiovasc Interv 2010; 3: 436 -41

Insights on FDA decision making Angiolillo DA, et al. Clin Pharmacol Ther 2011 89: 65 -74.

Insights on FDA decision making Four randomized, placebo-controlled, crossover PK/PD studies (n=282 healthy subjects) to investigate the interaction between: 1) Clopidogrel (300 -mg LD/75 -mg MD) & omeprazole (80 mg) administered simultaneously. 2) Clopidogrel (300 -mg LD/75 -mg MD) & omeprazole (80 mg) given 12 hours later. 3) Increasing the clopidogrel dose to 600 -mg LD/150 -mg MD & omeprazole (80 mg) administered simultaneously. 4) Clopidogrel (300 -mg LD/75 -mg MD) & pantoprazole (80 mg) administered simultaneously. Angiolillo DA, et al. Clin Pharmacol Ther 2011 89: 65 -74.

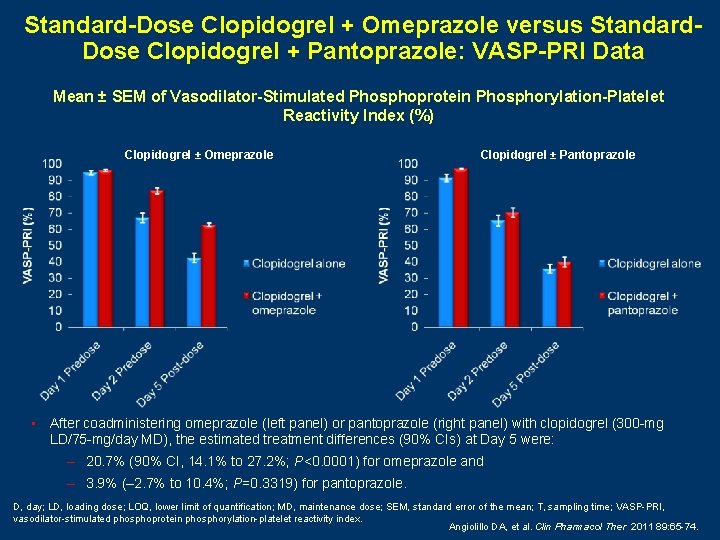
Standard-Dose Clopidogrel + Omeprazole versus Standard. Dose Clopidogrel + Pantoprazole: VASP-PRI Data Mean ± SEM of Vasodilator-Stimulated Phosphoprotein Phosphorylation-Platelet Reactivity Index (%) Clopidogrel ± Omeprazole Clopidogrel ± Pantoprazole • After coadministering omeprazole (left panel) or pantoprazole (right panel) with clopidogrel (300 -mg LD/75 -mg/day MD), the estimated treatment differences (90% CIs) at Day 5 were: – 20. 7% (90% CI, 14. 1% to 27. 2%; P<0. 0001) for omeprazole and – 3. 9% (– 2. 7% to 10. 4%; P=0. 3319) for pantoprazole. D, day; LD, loading dose; LOQ, lower limit of quantification; MD, maintenance dose; SEM, standard error of the mean; T, sampling time; VASP-PRI, vasodilator-stimulated phosphoprotein phosphorylation-platelet reactivity index. Angiolillo DA, et al. Clin Pharmacol Ther 2011 89: 65 -74.

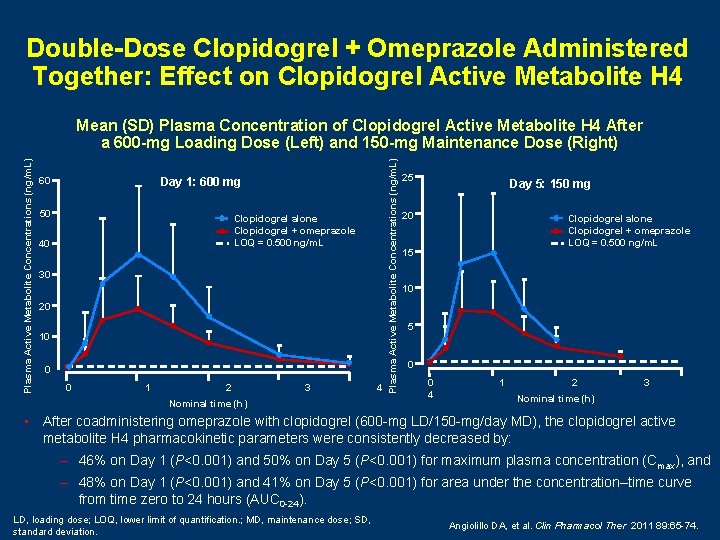
Double-Dose Clopidogrel + Omeprazole Administered Together: Effect on Clopidogrel Active Metabolite H 4 60 Day 1: 600 mg 50 Clopidogrel alone Clopidogrel + omeprazole LOQ = 0. 500 ng/m. L 40 30 20 10 0 0 1 2 3 Nominal time (h) 4 Plasma Active Metabolite Concentrations (ng/m. L) Mean (SD) Plasma Concentration of Clopidogrel Active Metabolite H 4 After a 600 -mg Loading Dose (Left) and 150 -mg Maintenance Dose (Right) 25 Day 5: 150 mg 20 Clopidogrel alone Clopidogrel + omeprazole LOQ = 0. 500 ng/m. L 15 10 5 0 0 4 1 2 3 Nominal time (h) • After coadministering omeprazole with clopidogrel (600 -mg LD/150 -mg/day MD), the clopidogrel active metabolite H 4 pharmacokinetic parameters were consistently decreased by: – 46% on Day 1 (P<0. 001) and 50% on Day 5 (P<0. 001) for maximum plasma concentration (Cmax), and – 48% on Day 1 (P<0. 001) and 41% on Day 5 (P<0. 001) for area under the concentration–time curve from time zero to 24 hours (AUC 0 -24). LD, loading dose; LOQ, lower limit of quantification. ; MD, maintenance dose; SD, standard deviation. Angiolillo DA, et al. Clin Pharmacol Ther 2011 89: 65 -74.

Drug-drug interactions Nat Rev Cardiol. 2009; 6: 392 -394

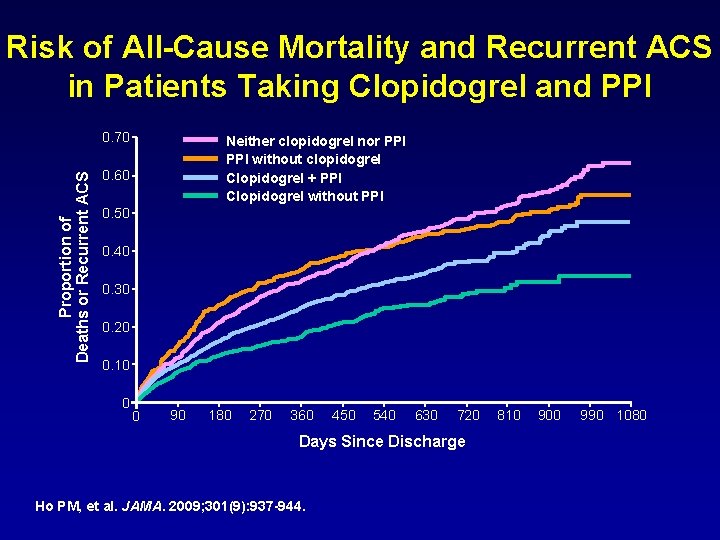
Risk of All-Cause Mortality and Recurrent ACS in Patients Taking Clopidogrel and PPI Proportion of Deaths or Recurrent ACS 0. 70 Neither clopidogrel nor PPI without clopidogrel Clopidogrel + PPI Clopidogrel without PPI 0. 60 0. 50 0. 40 0. 30 0. 20 0. 10 0 0 90 180 270 360 450 540 630 720 Days Since Discharge Ho PM, et al. JAMA. 2009; 301(9): 937 -944. 810 900 990 1080

TRITON-TIMI 38: Primary endpoint stratified by use of a PPI use at randomization (n= 4529) CV death, MI or stroke 14% 12% 10% Clopidogrel No PPI PPI No PPIPrasugrel 8% 6% 4% CLOPIDOGREL PPI vs no PPI: Adj HR 0. 94, 95% CI 0. 80 -1. 11 PRASUGREL PPI vs no PPI: Adj HR 1. 00, 95% CI 0. 84 -1. 20 2% 0% 0 100 200 Days 300 400 O'Donoghue ML et al. Lancet. 2009; 374: 989 -97.

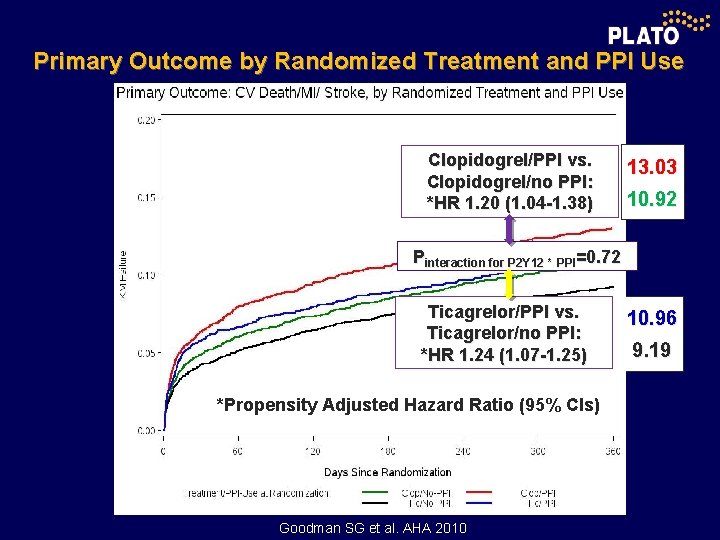
Primary Outcome by Randomized Treatment and PPI Use Clopidogrel/PPI vs. Clopidogrel/no PPI: *HR 1. 20 (1. 04 -1. 38) 13. 03 10. 92 Pinteraction for P 2 Y 12 * PPI=0. 72 Ticagrelor/PPI vs. Ticagrelor/no PPI: *HR 1. 24 (1. 07 -1. 25) *Propensity Adjusted Hazard Ratio (95% Cls) Goodman SG et al. AHA 2010 10. 96 9. 19

Clopidogrel and the Optimization of GI Events Trial – COGENT K–M Estimates of the Probability of Remaining Free of Primary Gastrointestinal Events HR: 0. 34; 95% CI: 0. 18 -0. 63 K–M Estimates of the Probability of Remaining Free of Primary Cardiovascular Events HR: 0. 99; 95% CI: 0. 68 -1. 44 Bhatt DL et al. N Engl J Med 2010; 363: 1909 -1917.

COGENT - Major Limitations 1. Trial interrupted prematurely for bankruptcy. 2. Not powered for CV events (“There was no a priori samplesize calculation or explicit noninferiority hypothesis for the CV endpoint”) 3. Definition of CV endpoint (CV death, non-fatal MI, ischemic stroke, or coronary revascularization) 4. Low CV event rate (109 overall events), thus broad CI (HR: 0. 99; 95% CI: 0. 68 -1. 44). Cannot exclude a clinically important increase in risk up to 44%. 5. Low major CV event rate (43 major events) –HR 1. 15 favoring placebo. 6. Authors conclusions: “Our results do not rule out a clinically meaningful difference in CV events due to the use of a PPI. ” 7. PPI used in COGENT: combo-pill (not available) with different PK profiles than commercially available omeprazole.
![Clopiodgrel FDA Box Warnings The Code of Federal Regulations 21 CFR 201 57 e Clopiodgrel FDA Box Warnings The Code of Federal Regulations (21 CFR 201. 57 [e])](https://slidetodoc.com/presentation_image_h/b9e2cd48f7b74a7315992015daeecca0/image-20.jpg)
Clopiodgrel FDA Box Warnings The Code of Federal Regulations (21 CFR 201. 57 [e]) requires that “labeling shall be revised to include a warning as soon as there is reasonable evidence of an association of a serious hazard with a drug; a causal relationship need not have been proved. Special problems, particularly those that may lead to death or serious injury, may be required by the Food and Drug Administration to be placed in a prominently displayed box. The boxed warning ordinarily shall be based on clinical data. ” Murphy S, Roberts R. "Black box" 101: How the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk. J Allergy Clin Immunol. 2006; 117: 34 -9.

The Statin Story 1. Only PD (no PK) interactions shown with CYP 3 A 4 interfering statins in the acute phase of treatment with standard (300 mg) loading-dose. 2. No PD interactions observed with high (600 mg) loading-dose or in maintenance phase (75 mg). 3. CYP 3 A 4 not as important on clopidogrel metabolism compared with CYP 2 C 19. 4. Clinical outcome data never concerning. 5. The PPI story is a different animal.

Clopidogrel – 2 C 19 specific PPI Interaction 1. PD and PK interaction between clopidogrel and 2 C 19 specific PPI is real! 2. The clinical implications of this interaction are still controversial / not fully defined. Therefore, this implies increased risk for harm (potentially lifethreatening), justifying the “boxed warning”.

There are reasons for warnings!

There are reasons for warnings!

Clopidogrel – 2 C 19 specific PPI Interaction 1. PD and PK interaction between clopidogrel and 2 C 19 specific PPI is real! 2. The clinical implications of this interaction are still controversial / not fully defined. Therefore, this implies increased risk for harm (potentially lifethreatening), justifying the “boxed warning”. 3. Don’t risk-the stakes are high! Other “roads” are available that are safer and equally efficacious (non 2 C 19 specific PPI, H 2 RA) to prevent bleeding. Follow the “boxed warning”!
 Dominick procopio
Dominick procopio Brian dominick
Brian dominick Dominick montgomery
Dominick montgomery Mdel fees
Mdel fees Iaems
Iaems Dr cr balance sheet
Dr cr balance sheet Welcome to our club gif
Welcome to our club gif Cushing & dolan
Cushing & dolan My isma lv
My isma lv Horizontal analysis example
Horizontal analysis example Envirepel energy
Envirepel energy Keller williams realty fees
Keller williams realty fees Portland state university tuition
Portland state university tuition Pcl5 molecular geometry
Pcl5 molecular geometry Dmc trade license fees
Dmc trade license fees Uncg tuition and fees
Uncg tuition and fees Non trading accounts
Non trading accounts Orbital virtual terminal
Orbital virtual terminal Tendertiger
Tendertiger Fidelity trust services fees
Fidelity trust services fees West hollywood rso
West hollywood rso Meed wced
Meed wced Mendel university fees
Mendel university fees Mount litera zee school besa
Mount litera zee school besa Fees earned debit or credit
Fees earned debit or credit Primera ley para sistemas abiertos
Primera ley para sistemas abiertos