DO NOW Pick up notes Get out Stoichiometry






- Slides: 14

DO NOW Pick up notes. Get out Stoichiometry of a Chemical Reaction lab and Off to the Races lab. Get out calculator and green periodic table.

MOLE TEST RETEST OPPORTUNITY: Available to anyone. Must attend TWO study sessions for the full time of each. Must be prepared to take the test BEFORE or AFTER school on Wednesday, February 15 at 7: 00 am or 3: 15 pm. Test cannot hurt your grade – only help.

OFF TO THE RACES RECAP

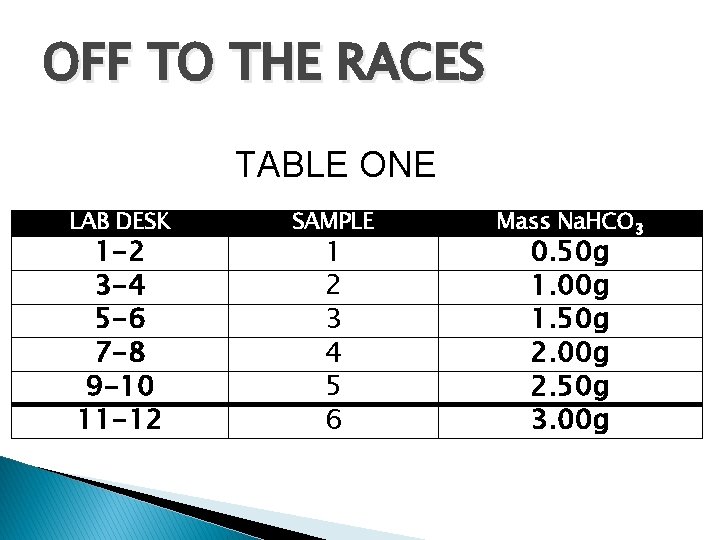
OFF TO THE RACES TABLE ONE LAB DESK 1 -2 3 -4 5 -6 7 -8 9 -10 11 -12 SAMPLE 1 2 3 4 5 6 Mass Na. HCO 3 0. 50 g 1. 00 g 1. 50 g 2. 00 g 2. 50 g 3. 00 g

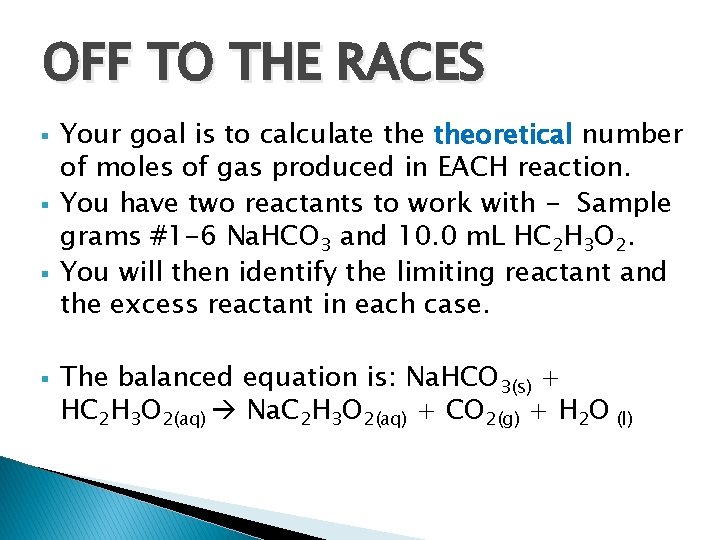
OFF TO THE RACES § § Your goal is to calculate theoretical number of moles of gas produced in EACH reaction. You have two reactants to work with - Sample grams #1 -6 Na. HCO 3 and 10. 0 m. L HC 2 H 3 O 2. You will then identify the limiting reactant and the excess reactant in each case. The balanced equation is: Na. HCO 3(s) + HC 2 H 3 O 2(aq) Na. C 2 H 3 O 2(aq) + CO 2(g) + H 2 O (l)

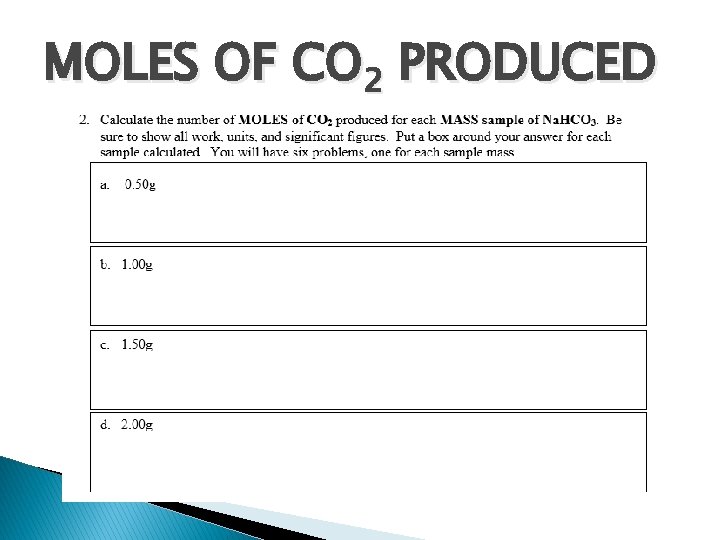
MOLES OF CO 2 PRODUCED

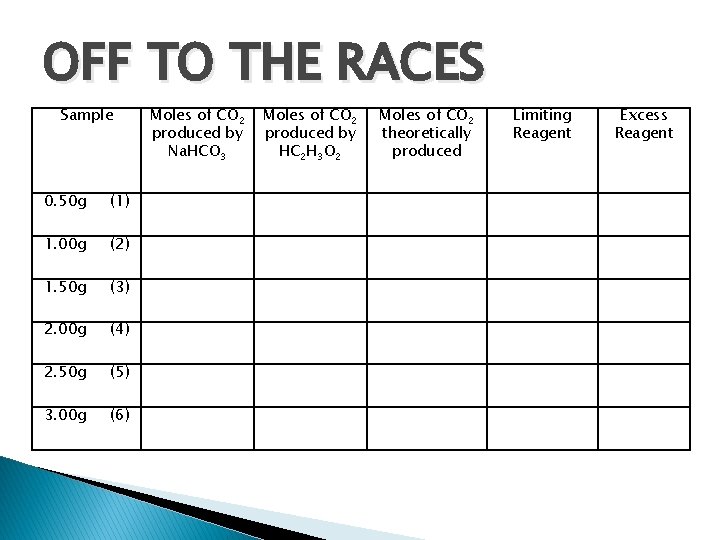
OFF TO THE RACES Sample 0. 50 g (1) 1. 00 g (2) 1. 50 g (3) 2. 00 g (4) 2. 50 g (5) 3. 00 g (6) Moles of CO 2 produced by Na. HCO 3 Moles of CO 2 produced by HC 2 H 3 O 2 Moles of CO 2 theoretically produced Limiting Reagent Excess Reagent

QUESTIONS?

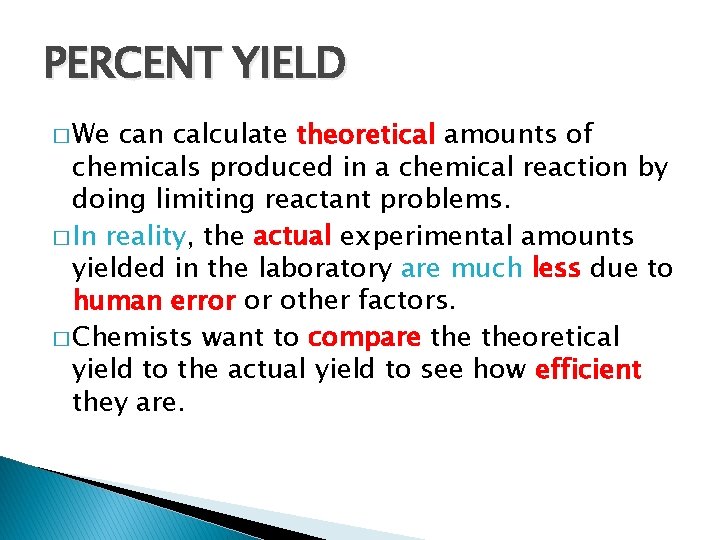
PERCENT YIELD � We can calculate theoretical amounts of chemicals produced in a chemical reaction by doing limiting reactant problems. � In reality, the actual experimental amounts yielded in the laboratory are much less due to human error or other factors. � Chemists want to compare theoretical yield to the actual yield to see how efficient they are.

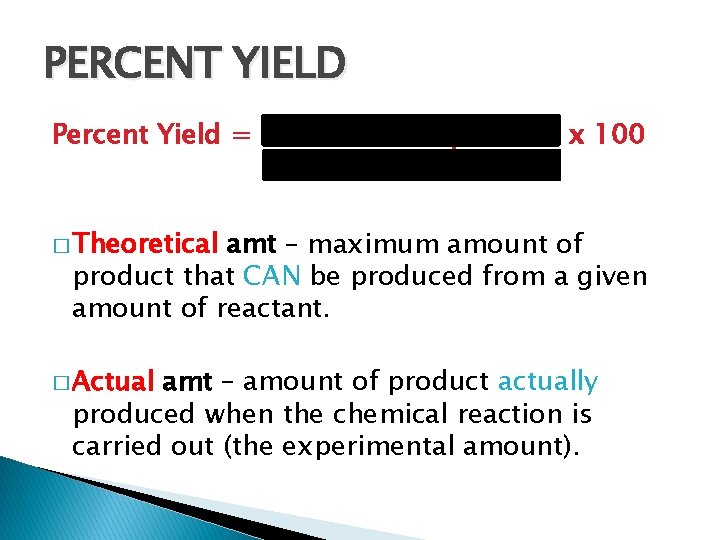
PERCENT YIELD Percent Yield = actual amt of product x 100 theoretical amt � Theoretical amt – maximum amount of product that CAN be produced from a given amount of reactant. � Actual amt – amount of product actually produced when the chemical reaction is carried out (the experimental amount).

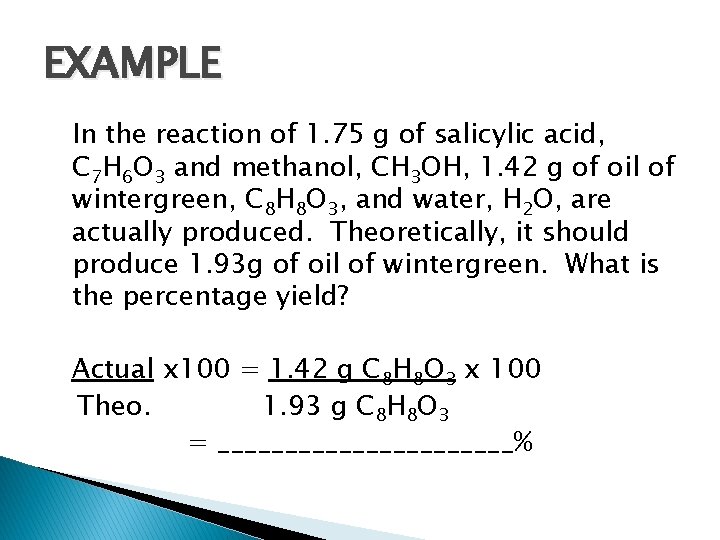
EXAMPLE In the reaction of 1. 75 g of salicylic acid, C 7 H 6 O 3 and methanol, CH 3 OH, 1. 42 g of oil of wintergreen, C 8 H 8 O 3, and water, H 2 O, are actually produced. Theoretically, it should produce 1. 93 g of oil of wintergreen. What is the percentage yield? Actual x 100 = 1. 42 g C 8 H 8 O 3 x 100 Theo. 1. 93 g C 8 H 8 O 3 = ___________%

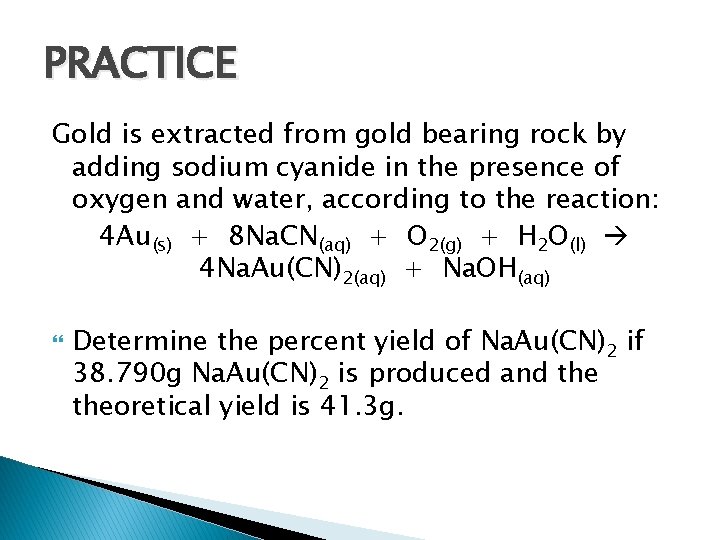
PRACTICE Gold is extracted from gold bearing rock by adding sodium cyanide in the presence of oxygen and water, according to the reaction: 4 Au(s) + 8 Na. CN(aq) + O 2(g) + H 2 O(l) 4 Na. Au(CN)2(aq) + Na. OH(aq) Determine the percent yield of Na. Au(CN)2 if 38. 790 g Na. Au(CN)2 is produced and theoretical yield is 41. 3 g.

NOW – STOICHIO LAB If you missed the explanation of #2 previously, you now know how to do Percent Yield. Finish and turn in the lab by the end of the period.

NOW Work on the Limiting Reactant and Percent Yield handout – due tomorrow. Off to the Races lab is also due tomorrow.