DISCOVER STUDY for HIV PreExposure Prophylaxis FTAF has



- Slides: 21

DISCOVER STUDY for HIV Pre-Exposure Prophylaxis: F/TAF has a more Rapid Onset and Longer Sustained Duration of HIV Protection Compared with F/TDF Christoph D. Spinner 1, Jason Brunetta 2, Peter Shalit 3, Maria Prins 4, Michelle Cespedes 5, Diana Brainard 6, Moupali Das 6, Scott Mc. Callister 6, Sophia R. Majeed 6, Anita Mathias 6, Ramin Ebrahimi 6, Frederick Cruickshank 8, Jason Halperin 9, Jay E. Gladstein 10, and Patrick W. G. Mallon 11 1 Technical University Munich, Germany; 2 Maple Leaf Medical Clinic, Toronto, Canada; 3 University of Washington School of Medicine, Seattle, USA; 4 Public Health Service of Amsterdam (Geneeskundige en Gezondheidsdienst Amsterdam), Netherlands; 5 Icahn School of Medicine at Mount Sinai, New York, USA; 6 Gilead Sciences, Inc. , Foster City, California, USA; 7 University of Colorado, Denver, CO, 8 Novant Health Presbyterian Medical Center, NC, USA; 9 Crescent. Care Health and Wellness Center, New Orleans, Louisiana, USA; 10 Global Healthcare Los Angeles, California, USA; 11 University College Dublin School of Medicine, Ireland Tuesday, July 23 IAS 2019, Mexico City

Thank You! Study participants, partners, families AUSTRIA B Haas A Rieger CANADA J Brunetta JJ de Wet B Trottier J Szabo C Tremblay DENMARK J Gerstoft G Kronborg C Larsen D Larsen FRANCE E Cua J-M Molina P Philibert G Pialoux GERMANY H Jessen G Knecht I Krznaric C Spinner IRELAND C Bergin P Mallon ITALY A Antinori A Lazzarin NETHERLANDS M Prins SPAIN J Coll M Crespo J del Romero Gerrero D Podzamczer Investigators and site staff UK V Apea A Clarke O Dosekun R Gilson S Kegg C Leen N Nwokolo F Post I Reeves G Schembri S Taylor US D Asmuth A Avery P Benson M Berhe I Brar C Brinson JH Burack T Campbell M Cespedes M Coleman CM Creticos GE Crofoot FA Cruickshank E Daar E De. Jesus Community advisors W Dinges S Doblecki-Lewis T Donovan J Flamm JE Gallant J Gladstein RM Grant R Grossberg J Halperin WD Hardy CB Hare S Hassler R Hengel K Henry T Hodge S Hosek M Iandorio A La. Marca C Lucasti S Mannheimer CT Martorell M Markowitz K Mayer A Mills S Morris K Mounzer O Ogbuagu O Osiyemi A Petroll J Phoenix MN Ramgopal B Rashbaum GJ Richmond PJ Ruane L Salazar AJ Scarsella M Scott P Shalit JL Stephens MA Thompson G Voskuhl BH Wade DA Wohl K Workowski B Young 2

Disclosures ¨ Dr. Spinner has received: – Research grants from Gilead, Janssen-Cilag, and Vii. V Healthcare – Honoraria for speakers bureau and/or advisory boards from Gilead, Abb. Vie, Janssen, MSD, Teva/Hexal, and Vii. V Healthcare ¨ This study was sponsored by Gilead Sciences 3

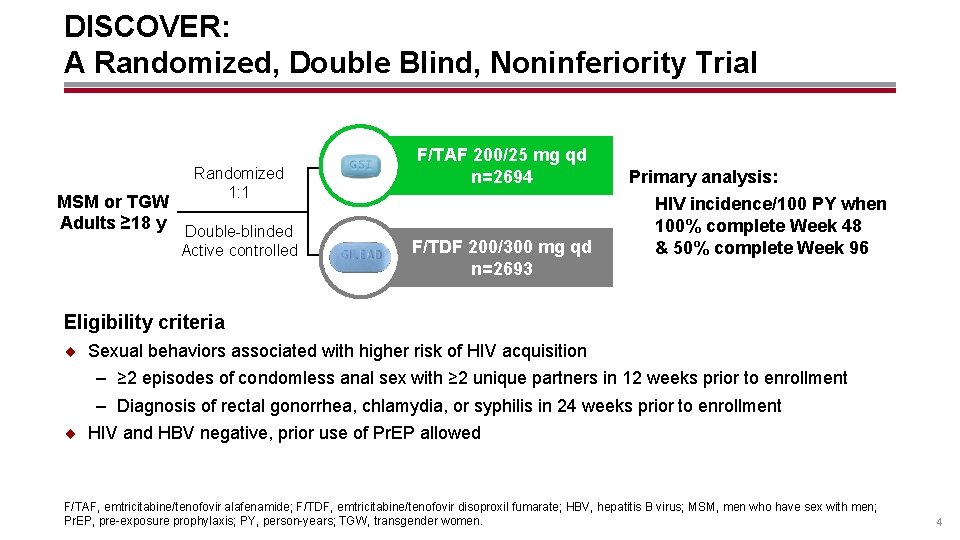
DISCOVER: A Randomized, Double Blind, Noninferiority Trial Randomized 1: 1 MSM or TGW Adults ≥ 18 y Double-blinded Active controlled F/TAF 200/25 mg qd n=2694 F/TDF 200/300 mg qd n=2693 Primary analysis: HIV incidence/100 PY when 100% complete Week 48 & 50% complete Week 96 Eligibility criteria ¨ Sexual behaviors associated with higher risk of HIV acquisition – ≥ 2 episodes of condomless anal sex with ≥ 2 unique partners in 12 weeks prior to enrollment – Diagnosis of rectal gonorrhea, chlamydia, or syphilis in 24 weeks prior to enrollment ¨ HIV and HBV negative, prior use of Pr. EP allowed F/TAF, emtricitabine/tenofovir alafenamide; F/TDF, emtricitabine/tenofovir disoproxil fumarate; HBV, hepatitis B virus; MSM, men who have sex with men; Pr. EP, pre-exposure prophylaxis; PY, person-years; TGW, transgender women. 4

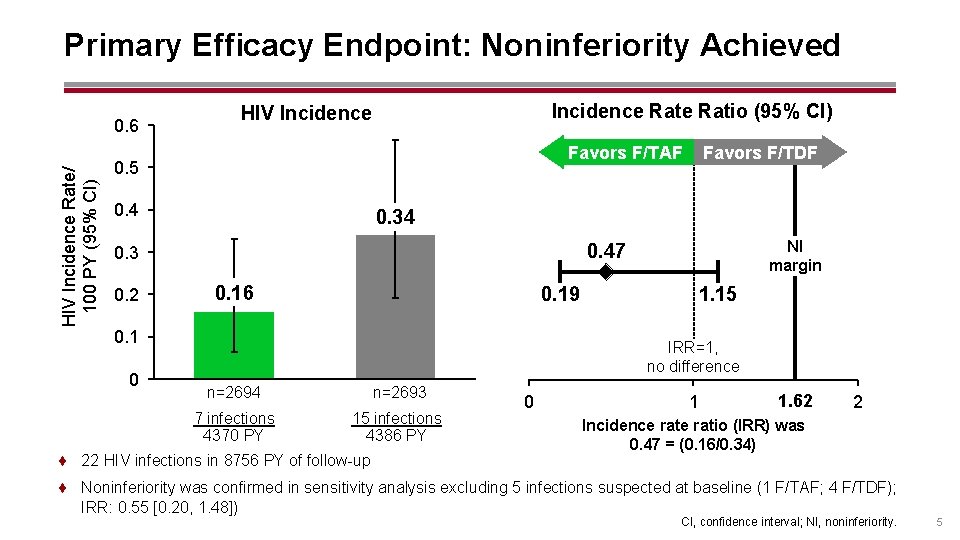
Primary Efficacy Endpoint: Noninferiority Achieved HIV Incidence Rate/ 100 PY (95% CI) 0. 6 Incidence Ratio (95% CI) HIV Incidence Favors F/TAF 0. 5 0. 4 0. 34 0. 16 0. 19 0. 1 0 NI margin 0. 47 0. 3 0. 2 Favors F/TDF 1. 15 IRR=1, no difference n=2694 n=2693 7 infections 4370 PY 15 infections 4386 PY ¨ 22 HIV infections in 8756 PY of follow-up 0 1 1. 62 2 Incidence ratio (IRR) was 0. 47 = (0. 16/0. 34) ¨ Noninferiority was confirmed in sensitivity analysis excluding 5 infections suspected at baseline (1 F/TAF; 4 F/TDF); IRR: 0. 55 [0. 20, 1. 48]) CI, confidence interval; NI, noninferiority. 5

Were the Fewer HIV Infections in F/TAF Due to Chance? ¨ The incidence ratio (IRR) was 0. 47 (95% CI 0. 19, 1. 15) reflecting that the point estimate of the HIV incidence rate was 53% lower in the F/TAF arm compared with F/TDF ¨ Probability of the above result due to chance is 3 in 1, 000 ¨ Post-hoc Bayesian analysis calculated a 96% (95% credible interval) probability that F/TAF is more effective than F/TDF ¨ We examined sexual behavior, STI incidence, adherence, and PK to evaluate any differences between F/TAF and F/TDF which could explain the fewer infections in the F/TAF arm 6

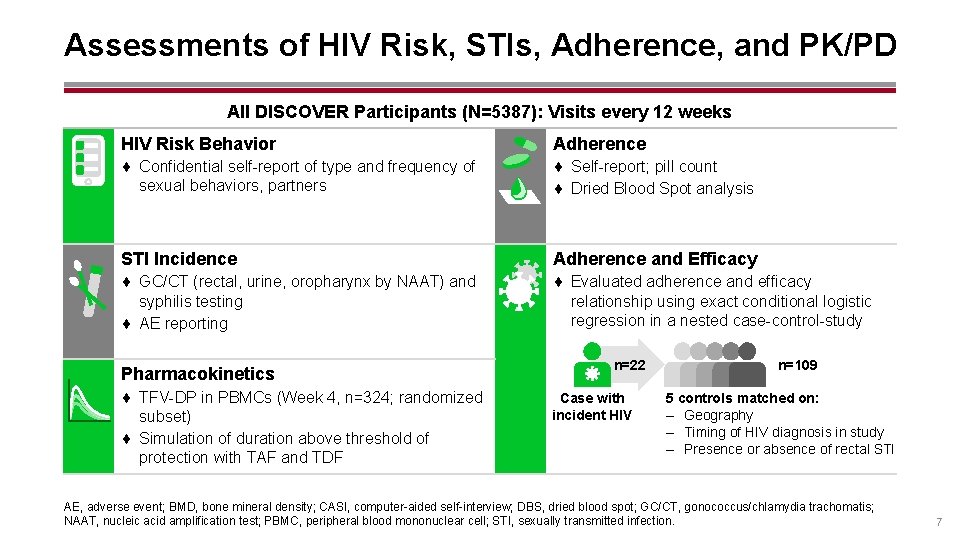
Assessments of HIV Risk, STIs, Adherence, and PK/PD All DISCOVER Participants (N=5387): Visits every 12 weeks HIV Risk Behavior Adherence ¨ Confidential self-report of type and frequency of sexual behaviors, partners ¨ Self-report; pill count ¨ Dried Blood Spot analysis STI Incidence Adherence and Efficacy ¨ GC/CT (rectal, urine, oropharynx by NAAT) and syphilis testing ¨ AE reporting ¨ Evaluated adherence and efficacy relationship using exact conditional logistic regression in a nested case-control-study Pharmacokinetics ¨ TFV-DP in PBMCs (Week 4, n=324; randomized subset) ¨ Simulation of duration above threshold of protection with TAF and TDF n=22 Case with incident HIV n=109 5 controls matched on: – Geography – Timing of HIV diagnosis in study – Presence or absence of rectal STI AE, adverse event; BMD, bone mineral density; CASI, computer-aided self-interview; DBS, dried blood spot; GC/CT, gonococcus/chlamydia trachomatis; NAAT, nucleic acid amplification test; PBMC, peripheral blood mononuclear cell; STI, sexually transmitted infection. 7

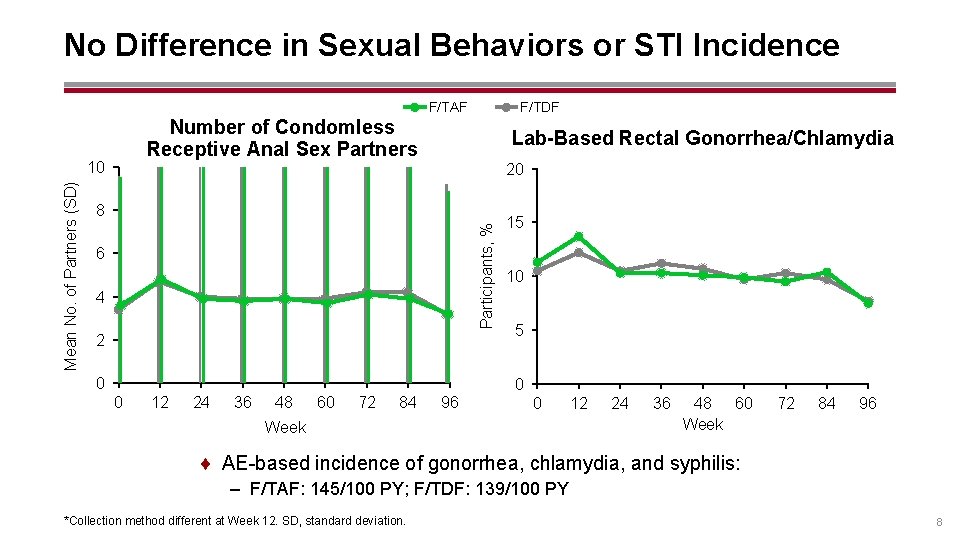
No Difference in Sexual Behaviors or STI Incidence F/TDF F/TAF Number of Condomless Receptive Anal Sex Partners 20 8 Participants, % Mean No. of Partners (SD) 10 Lab-Based Rectal Gonorrhea/Chlamydia 6 4 2 0 15 10 5 0 0 12 24 36 48 60 72 84 96 0 Week 12 24 36 48 60 Week 72 84 96 ¨ AE-based incidence of gonorrhea, chlamydia, and syphilis: – F/TAF: 145/100 PY; F/TDF: 139/100 PY *Collection method different at Week 12. SD, standard deviation. 8

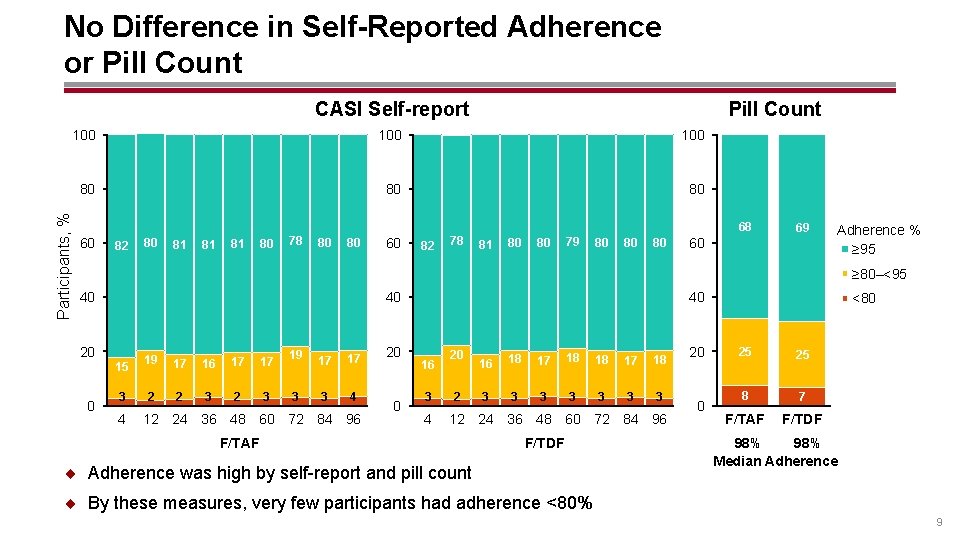
No Difference in Self-Reported Adherence or Pill Count CASI Self-report Pill Count 100 80 80 80 Participants, % 100 68 60 82 80 81 81 81 80 78 80 80 60 82 78 81 80 80 79 80 80 80 69 60 Adherence % ≥ 95 ≥ 80–<95 40 40 20 15 0 3 4 19 17 16 17 17 2 2 3 19 17 17 3 3 4 12 24 36 48 60 72 84 96 20 0 40 16 3 4 20 2 16 18 17 18 3 3 3 3 12 24 36 48 60 72 84 96 F/TAF F/TDF ¨ Adherence was high by self-report and pill count 20 0 <80 25 25 8 7 F/TAF F/TDF 98% Median Adherence ¨ By these measures, very few participants had adherence <80% 9

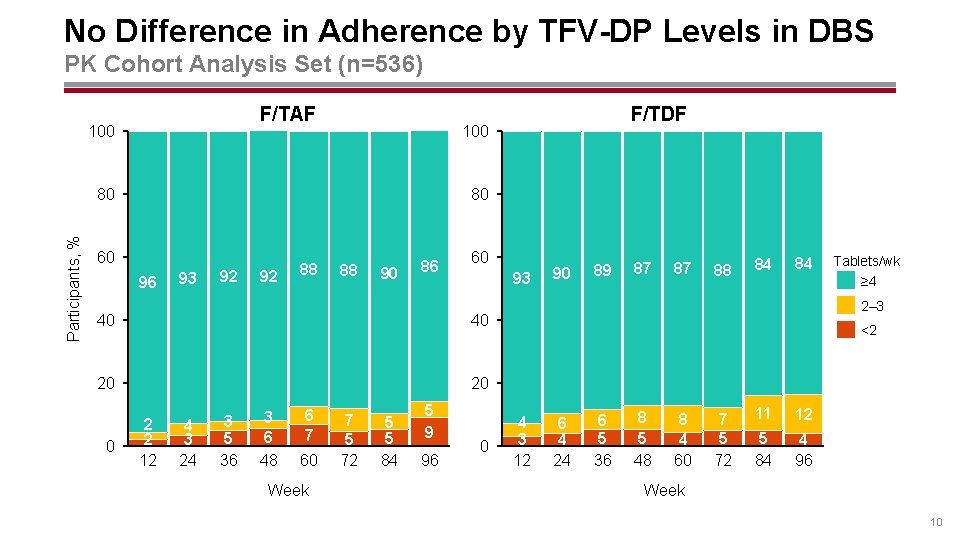
No Difference in Adherence by TFV-DP Levels in DBS PK Cohort Analysis Set (n=536) F/TAF 100 Participants, % 80 80 60 96 93 92 92 88 88 90 86 60 93 40 40 20 20 0 F/TDF 2 2 12 4 3 24 3 5 36 3 6 48 6 7 60 Week 7 5 72 5 5 84 5 9 96 0 90 89 87 87 88 84 84 Tablets/wk ≥ 4 2– 3 <2 4 3 12 6 4 24 6 5 36 8 5 48 8 4 60 7 5 72 11 12 5 84 4 96 Week 10

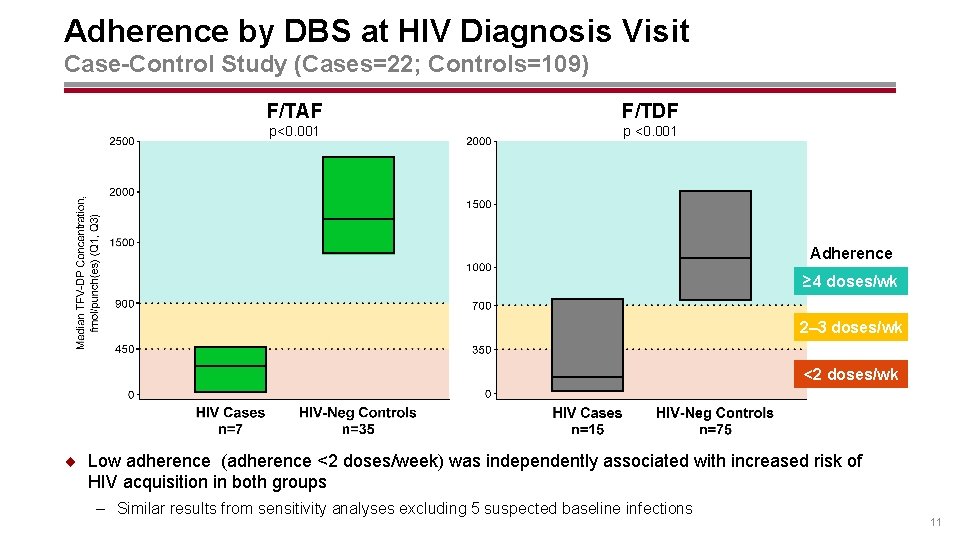
Adherence by DBS at HIV Diagnosis Visit Case-Control Study (Cases=22; Controls=109) F/TAF F/TDF p<0. 001 p <0. 001 Adherence ≥ 4 doses/wk 2– 3 doses/wk <2 doses/wk ¨ Low adherence (adherence <2 doses/week) was independently associated with increased risk of HIV acquisition in both groups – Similar results from sensitivity analyses excluding 5 suspected baseline infections 11

Summary of Behaviors No Differences Between Arms in HIV Risk, STIs, or Adherence F/TAF F/TDF ¨ There were no differences in HIV risk behavior, prevalence or incidence of STIs, or adherence by pill count, self-report, or dried blood spot between F/TAF and F/TDF ¨ Low adherence was strongly associated with HIV acquisition in both arms ¨ Next we examined pharmacokinetic differences between F/TAF and F/TDF 12

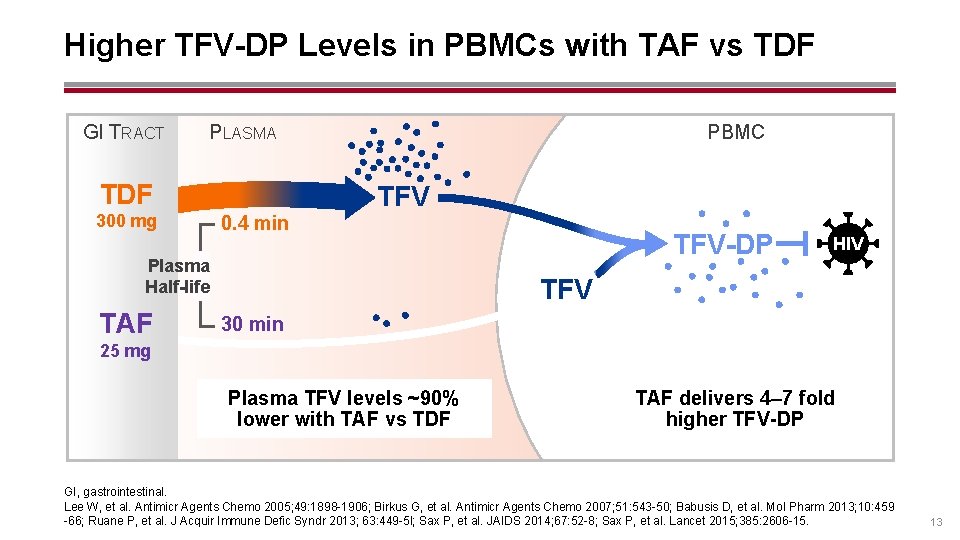
Higher TFV-DP Levels in PBMCs with TAF vs TDF GI TRACT PLASMA TDF 300 mg 0. 4 min PBMC TFV Plasma Half-life TAF TFV-DP HIV TFV 30 min 25 mg Plasma TFV levels ~90% lower with TAF vs TDF TAF delivers 4– 7 fold higher TFV-DP GI, gastrointestinal. Lee W, et al. Antimicr Agents Chemo 2005; 49: 1898 -1906; Birkus G, et al. Antimicr Agents Chemo 2007; 51: 543 -50; Babusis D, et al. Mol Pharm 2013; 10: 459 -66; Ruane P, et al. J Acquir Immune Defic Syndr 2013; 63: 449 -5 l; Sax P, et al. JAIDS 2014; 67: 52 -8; Sax P, et al. Lancet 2015; 385: 2606 -15. 13

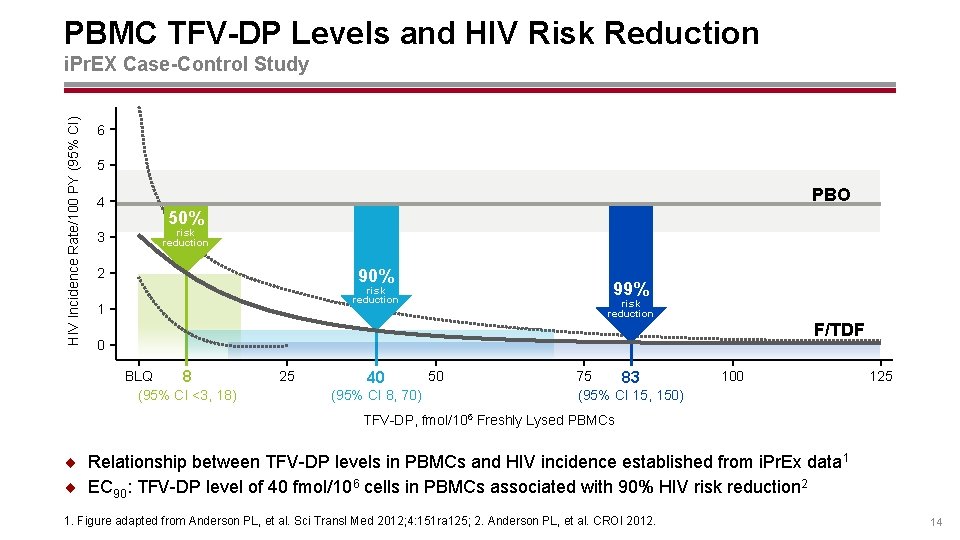
PBMC TFV-DP Levels and HIV Risk Reduction HIV Incidence Rate/100 PY (95% CI) i. Pr. EX Case-Control Study 6 5 4 3 PBO 50% risk reduction 90% 2 99% risk reduction 1 risk reduction F/TDF 0 BLQ 8 (95% CI <3, 18) 25 40 (95% CI 8, 70) 50 75 83 (95% CI 15, 150) 100 125 TFV-DP, fmol/106 Freshly Lysed PBMCs ¨ Relationship between TFV-DP levels in PBMCs and HIV incidence established from i. Pr. Ex data 1 ¨ EC 90: TFV-DP level of 40 fmol/106 cells in PBMCs associated with 90% HIV risk reduction 2 1. Figure adapted from Anderson PL, et al. Sci Transl Med 2012; 4: 151 ra 125; 2. Anderson PL, et al. CROI 2012. 14

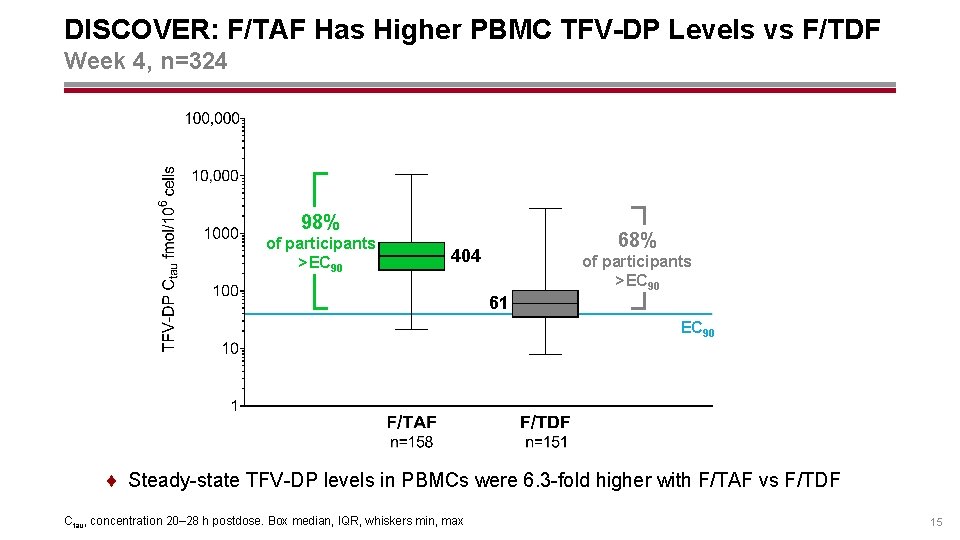
DISCOVER: F/TAF Has Higher PBMC TFV-DP Levels vs F/TDF Week 4, n=324 98% of participants >EC 90 68% 404 of participants >EC 90 61 EC 90 ¨ Steady-state TFV-DP levels in PBMCs were 6. 3 -fold higher with F/TAF vs F/TDF Ctau, concentration 20– 28 h postdose. Box median, IQR, whiskers min, max 15

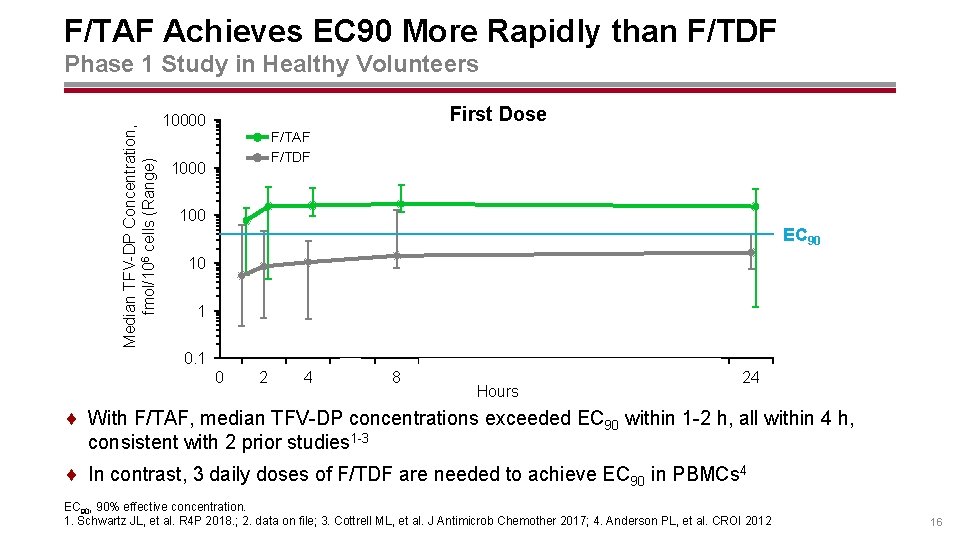
F/TAF Achieves EC 90 More Rapidly than F/TDF Median TFV-DP Concentration, fmol/106 cells (Range) Phase 1 Study in Healthy Volunteers First Dose 10000 F/TAF F/TDF 1000 100 EC 90 10 1 0 2 4 6 8 10 12 14 Hours 16 18 20 22 24 ¨ With F/TAF, median TFV-DP concentrations exceeded EC 90 within 1 -2 h, all within 4 h, consistent with 2 prior studies 1 -3 ¨ In contrast, 3 daily doses of F/TDF are needed to achieve EC 90 in PBMCs 4 EC 90, 90% effective concentration. 1. Schwartz JL, et al. R 4 P 2018. ; 2. data on file; 3. Cottrell ML, et al. J Antimicrob Chemother 2017; 4. Anderson PL, et al. CROI 2012 16

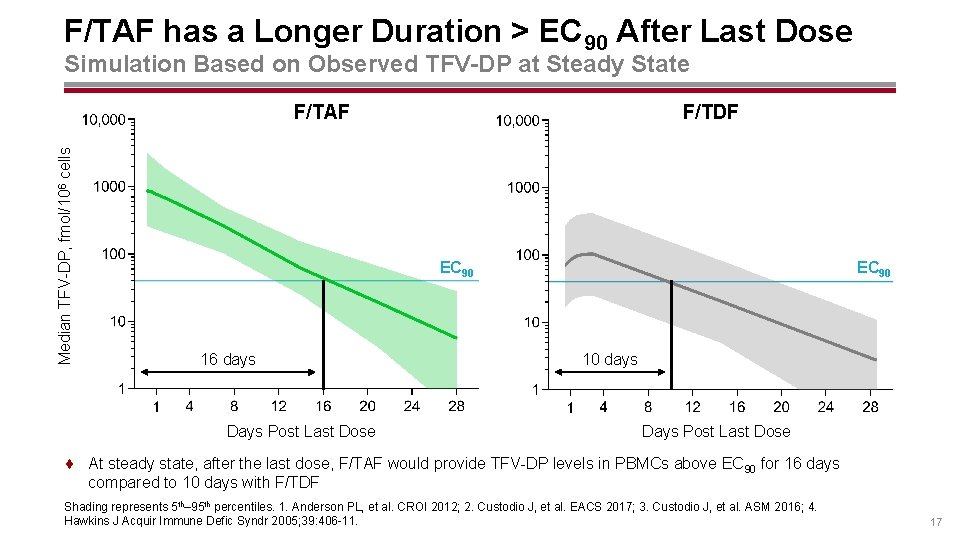
F/TAF has a Longer Duration > EC 90 After Last Dose Simulation Based on Observed TFV-DP at Steady State Median TFV-DP, fmol/106 cells F/TAF F/TDF EC 90 16 days Days Post Last Dose EC 90 10 days Days Post Last Dose ¨ At steady state, after the last dose, F/TAF would provide TFV-DP levels in PBMCs above EC 90 for 16 days compared to 10 days with F/TDF Shading represents 5 th– 95 th percentiles. 1. Anderson PL, et al. CROI 2012; 2. Custodio J, et al. EACS 2017; 3. Custodio J, et al. ASM 2016; 4. Hawkins J Acquir Immune Defic Syndr 2005; 39: 406 -11. 17

Conclusions: F/TAF Has a More Rapid Onset and Longer Sustained Duration of Protection than F/TDF ¨ Noninferiority of F/TAF to F/TDF was established by the lower HIV incidence of F/TAF compared with F/TDF – Risk behavior, STIs, and Adherence were similar between arms ¨ HIV prevention efficacy PK parameters differed between F/TAF vs F/TDF: – TFV-DP levels in PBMCS were 6. 3 fold higher with F/TAF vs F/TDF – 98% in the F/TAF arm are above EC 90 compared with 68% in F/TDF arm – F/TAF achieved EC 90 within 1– 2 hrs of first dose vs 3 days of daily doses of F/TDF – F/TAF expected to remain above EC 90 for 16 days vs 10 days for F/TDF ¨ The more rapid onset and longer duration of protection may be the most probable explanation for the higher prevention efficacy of F/TAF ¨ F/TAF is a safer, potentially more efficacious option than F/TDF for prevention of HIV 18

BACK-UPS 19

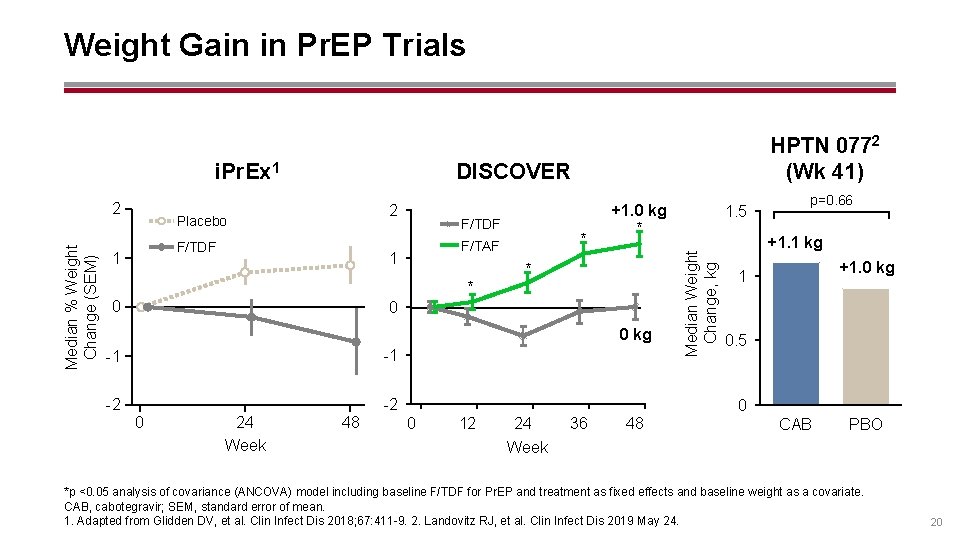
Weight Gain in Pr. EP Trials Median % Weight Change (SEM) 2 DISCOVER 2 Placebo F/TDF 1 F/TDF * F/TAF 1 * 0 +1. 0 kg * * 0 0 kg -1 -1 -2 -2 0 24 Week 48 1. 5 Median Weight Change, kg i. Pr. Ex 1 HPTN 0772 (Wk 41) p=0. 66 +1. 1 kg +1. 0 kg 1 0. 5 0 0 12 24 36 48 CAB PBO Week *p <0. 05 analysis of covariance (ANCOVA) model including baseline F/TDF for Pr. EP and treatment as fixed effects and baseline weight as a covariate. CAB, cabotegravir; SEM, standard error of mean. 1. Adapted from Glidden DV, et al. Clin Infect Dis 2018; 67: 411 -9. 2. Landovitz RJ, et al. Clin Infect Dis 2019 May 24. 20

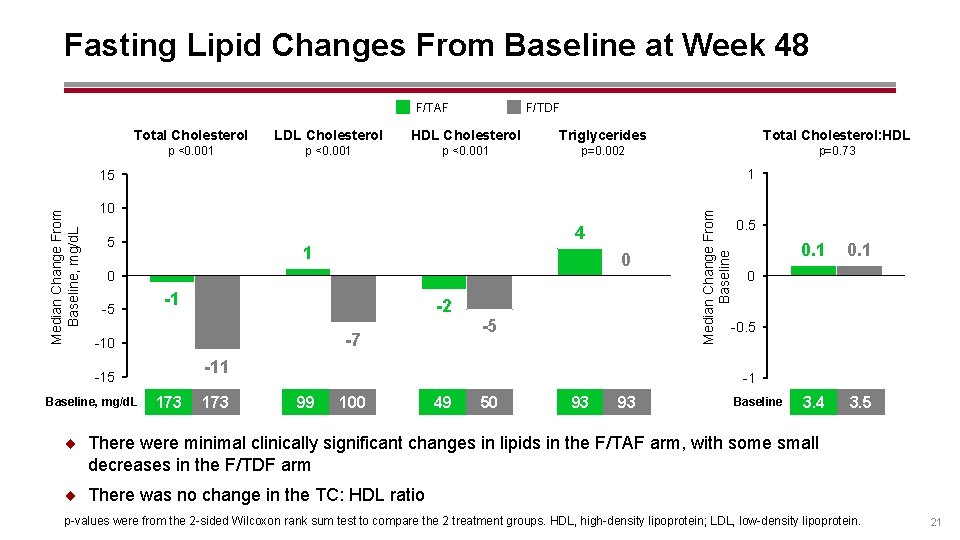
Fasting Lipid Changes From Baseline at Week 48 F/TDF F/TAF Total Cholesterol LDL Cholesterol HDL Cholesterol Triglycerides Total Cholesterol: HDL p <0. 001 p=0. 002 p=0. 73 1 10 4 5 1 0 0 -5 -1 -2 -10 173 0. 5 0. 1 3. 4 3. 5 0 -0. 5 -11 -15 Baseline, mg/d. L -5 -7 Median Change From Baseline, mg/d. L 15 -1 99 100 49 50 93 93 Baseline ¨ There were minimal clinically significant changes in lipids in the F/TAF arm, with some small decreases in the F/TDF arm ¨ There was no change in the TC: HDL ratio p-values were from the 2 -sided Wilcoxon rank sum test to compare the 2 treatment groups. HDL, high-density lipoprotein; LDL, low-density lipoprotein. 21