Definition of Air Pollution Air pollution occurs when





























- Slides: 32

Definition of Air Pollution Air pollution occurs when the air contains gases, dust, fumes or odour in harmful amounts. That is, amounts which could be harmful to the health or comfort of humans and animals or which could damage to plants and materials. The substances that cause air pollution are called pollutants.

Air Pollution is the human introduction into the atmosphere of chemicals, particulates, or biological materials that cause harm or discomfort to humans or other living organisms, or damage the environment. Air pollution causes deaths and respiratory disease.

Sources of Pollutants The two main sources of pollutants in urban areas are transportation (predominantly automobiles) and fuel combustion in stationary sources, including residential, commercial, and industrial heating and cooling and coal-burning power plants.

Motor vehicles produce high levels of carbon monoxides (CO) and a major source of hydrocarbons (HC) and nitrogen oxides (NOx). Whereas, fuel combustion in stationary sources is the dominant source of sulfur dioxide (SO 2).

Carbon Dioxide - CO 2 Carbon dioxide (CO 2) is one of the major pollutants in the atmosphere. Major sources of CO 2 are fossil fuels burning and deforestation. CO 2 a greenhouse gas emitted from combustion.

Carbon Monoxide -CO Carbon monoxide is colourless, odourless, non-irritating but very poisonous gas. It is a product by incomplete combustion of fuel such as natural gas, coal or wood. Vehicular exhaust is a major source of carbon monoxide.

N 0 X - nitric oxide (N 0) and nitrogen dioxide (N 02) l l l Natural component of the Earth's atmosphere. Important in the formation of both acid precipitation and photochemical smog (ozone), and causes nitrogen loading. Comes from the burning of biomass and fossil fuels. 30 to 50 million tons per year from human activities, and natural 10 to 20 million tons per year. Average residence time in the atmosphere is days. Has a role in reducing stratospheric ozone.

Sulfur Oxides - SOx Sulfur oxides (SOx) especially sulfur dioxide are emitted from burning of coal and oil. At sufficiently high concentrations, sulfur dioxide irritates the upper respiratory tract of human beings because potential effect of sulfur dioxide is to make breathing more difficult by causing the finer air tubes of the lung to constrict.

Chlorofluorocarbons (CFCs) CFCs are lowering the average concentration of ozone in the stratosphere. l Spray cans, discarded or leaking refrigeration and air conditioning equipment, and the burning plastic foam products release the CFCs into the atmosphere. Depending on the type, CFCs stay in the atmosphere from 22 to 111 years. l

Smog This smog is created by burning coal and heavy oil that contain sulfur impurities in power plants, industrial plants, etc. . . The smog consists mostly of a mixture of sulfur dioxide and fog. Suspended droplets of sulfuric acid are formed from some of the sulfur dioxide, and a variety of suspended solid particles. This smog is common during the winter in cities such as London, Chicago, Pittsburgh. When these cities burned large amounts of coal and heavy oil without control of the output, large-scale problems were witnessed.

In 1952 London, England, 4, 000 people died as a result of this form of fog. Today coal and heavy oil are burned only in large boilers and with reasonably good control or tall smokestacks so that industrial smog is less of a problem.

Smog can form in almost any climate where industries or cities release large amounts of air pollution.

Volatile Organic Compounds (VOC), such as hydrocarbon fuel vapors and solvents. Toxic metals such as lead, cadmium and copper. Ammonia emitted from agricultural processes. Odors such as from garbage, sewage and industrial processes. Radioactive pollutants.

Secondary Pollutants: Ground level ozone (O 3) formed from NOx and VOC. Peroyacetyl (PAN) similarly formed from NOx and VOC.

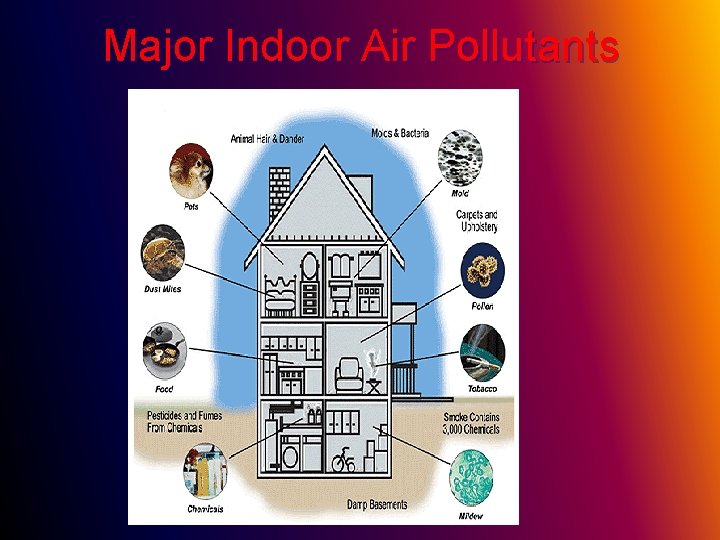
Indoor Air Pollutions from power plants, cars, and other transportation is a well-known contributor to outdoor air pollution. But indoor air pollution is often worse; it can be up to 10 times worse for you than the air outside.

Indoor pollution sources release chemicals, micro-organisms, and particulates into the air l Inadequate ventilation to dilute the pollutants. l Inadequate filtration to remove microorganisms and particulates l

Major Indoor Air Pollutants

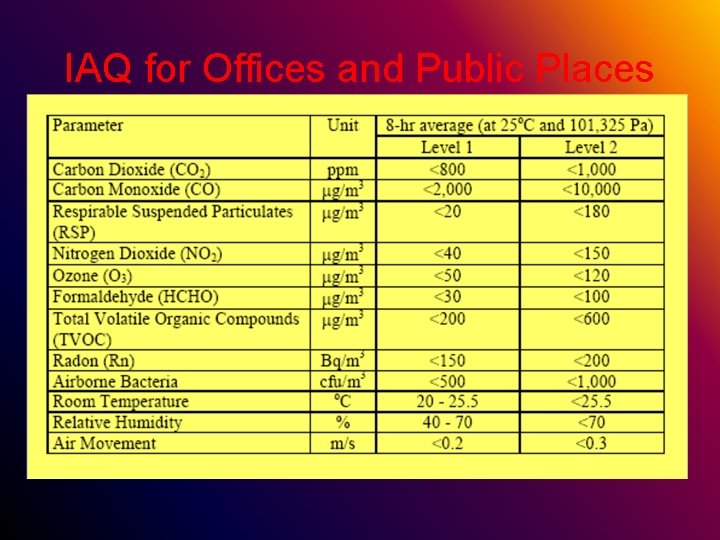
IAQ for Offices and Public Places

Health Effects Air pollution can affect our health in many ways with both short-term and long-term effects. Examples of short-term effects include irritation to the eyes, nose and throat, and upper respiratory infections such as bronchitis and pneumonia. Other symptoms can include headaches, nausea, and allergic reactions.

Long-term health effects can include chronic respiratory disease, lung cancer, heart disease, and even damage to the brain, nerves, liver, or kidneys. It is estimated that half a million people die prematurely every year in the United States as a result of smoking cigarettes.

Environmental Impacts Pollutants such as sulfur dioxide, nitrogen oxides, ozone and peroxyacl nitrates (PANs), cause direct damage to leaves of crop plants and trees when they enter leaf pores (stomates).

Trees play an important role in producing oxygen from carbon dioxide.

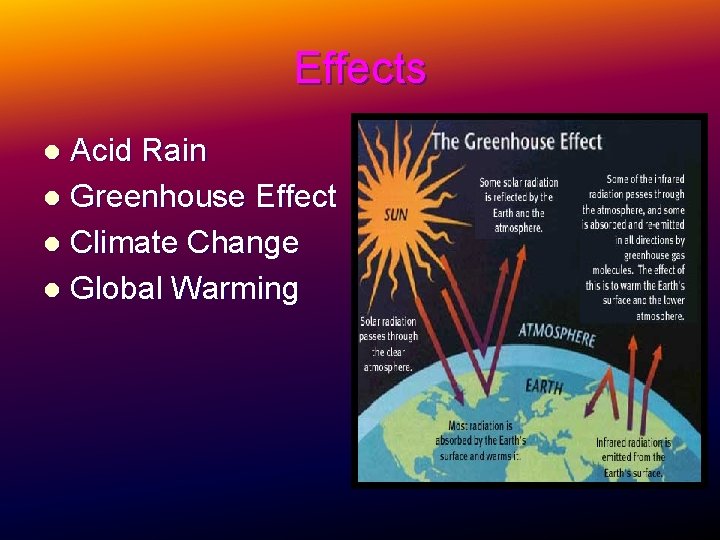
Effects Acid Rain l Greenhouse Effect l Climate Change l Global Warming l

With the destruction and burning of the rain forests more and more CO 2 is being released into the atmosphere. Trees play an important role in producing oxygen from carbon dioxide. This process is called photosynthesis which all plants go though but some yield more and some less oxygen. As long as no more wood is burnt than is reproduced by the forests, no change in atmospheric CO 2 concentration will result.

Acid Rain "Acid rain" is a broad term referring to a mixture of wet and dry deposition (deposited material) from the atmosphere containing higher than normal amounts of nitric and sulfuric acids. The precursors, or chemical forerunners, of acid rain formation result from both natural sources, such as volcanoes and decaying vegetation, and man-made sources, primarily emissions of sulfur dioxide (SO 2) and nitrogen oxides (NOx) resulting from fossil fuel combustion.

Wet Deposition Wet deposition refers to acidic rain, fog, and snow. If the acid chemicals in the air are blown into areas where the weather is wet, the acids can fall to the ground in the form of rain, snow, fog, or mist. As this acidic water flows over and through the ground, it affects a variety of plants and animals. The strength of the effects depends on several factors, including how acidic the water is; the chemistry and buffering capacity of the soils involved; and the types of fish, trees, and

Dry Deposition In areas where the weather is dry, the acid chemicals may become incorporated into dust or smoke and fall to the ground through dry deposition, sticking to the ground, buildings, homes, cars, and trees. Dry deposited gases and particles can be washed from these surfaces by rainstorms, leading to increased runoff. This runoff water makes the resulting mixture more acidic. About half of the acidity in the atmosphere falls back to earth through dry deposition

Greenhouse Effect The greenhouse effect is unquestionably real and helps to regulate the temperature of our planet. It is essential for life on Earth and is one of Earth's natural processes. It is the result of heat absorption by certain gases in the atmosphere (called greenhouse gases because they effectively 'trap' heat in the lower atmosphere) and re-radiation downward of some of that heat.

Water vapor is the most abundant greenhouse gas, followed by carbon dioxide and other trace gases. Without a natural greenhouse effect, the temperature of the Earth would be about zero degrees F (-18 C) instead of its present 57 F (14 C)

So, the concern is not with the fact that we have a greenhouse effect, but whether human activities are leading to an enhancement of the greenhouse effect by the emission of greenhouse gases through fossil fuel combustion and deforestation.

Climate Change and. Global Warming Global warming is the increase in the average temperature of the Earth's nearsurface air and oceans since the midtwentieth century, and its projected continuation. Increasing global temperature will cause sea level to rise, and is expected to increase the intensity of extreme weather events and to change the amount and pattern of precipitation.

 Chapter 12 air section 1
Chapter 12 air section 1 Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Air pollution definition
Air pollution definition Hubungan air dengan tanah
Hubungan air dengan tanah Effect on human health of land pollution
Effect on human health of land pollution Controlling measures of noise pollution
Controlling measures of noise pollution Land water and air pollution
Land water and air pollution Introduction about air pollution
Introduction about air pollution Air pollution effects to plants
Air pollution effects to plants Contents of air pollution
Contents of air pollution Aims and objectives of air pollution
Aims and objectives of air pollution Main cause of air pollution
Main cause of air pollution What is secondary pollutant
What is secondary pollutant Stationary and mobile sources of air pollution
Stationary and mobile sources of air pollution Air pollution box model example
Air pollution box model example General effects of air pollution
General effects of air pollution Air pollution consequences
Air pollution consequences Erg (air pollution control) ltd
Erg (air pollution control) ltd Air pollution
Air pollution Objectives of air pollution
Objectives of air pollution Air pollution
Air pollution Air pollution
Air pollution Air pollution class 9
Air pollution class 9 Aims and objectives of environmental pollution
Aims and objectives of environmental pollution Two sources of air pollution
Two sources of air pollution 5 effects of air pollution
5 effects of air pollution Fixed box model air pollution
Fixed box model air pollution Northern sonoma county air pollution control district
Northern sonoma county air pollution control district Indoor air pollution examples
Indoor air pollution examples Air pollution simulator
Air pollution simulator Air pollution 2050
Air pollution 2050 Acid rain in canada
Acid rain in canada Air pollution control methods
Air pollution control methods