DAY 14 Sig figs Scientific Notation Day 15












- Slides: 17

DAY 14 – Sig figs / Scientific Notation Day 15 - Density LAB Day 16 – Measurement / sig fig quiz

Density Lab • Materials; – Cork stopper, Rubber stopper, Nut and bolt – Graduated cylinder, water Formula: D=M/V Data Table: MASS VOLUME Final (m. L) Cork Stopper Rubber Stopper Nut & Bolt Initial (m. L) DENSITY Volume

Scientific Notation What's the goal of Scientific Notation? – Condense the number that is written Example: What would you rather write – 0. 000000000456 Or – 4. 56 x 10 -18

Rules of Scientific Notation • It’s all about the decimal point! And power of 10! Example: 645, 000 1 st… move the decimal point so one # is to the left of it 6. 45000 2 nd… place “x 10” to the right 6. 45000 x 10 3 rd… count the number of spaces you moved the decimal point. 6. 45000 X 105 Almost Done!

• 6. 45000 X 105 Now get rid of the zeros 6. 45 X 105 Rules: #1. . moving the decimal point to the left… the exponent gets bigger #2… moving the decimal point to the right… the exponent gets smaller 64. 5 X 10… what's the exponent 6. 45 X 104

Adding Exponents Rules: 1 st… exponents need to be the same. Move the decimal point until the two numbers have the same exponent 2 nd… add OR subtract the numbers… not the exponents. Example: Add the following #s…Follow the Rules 6. 45 x 105 3. 11 x 104 Answer: 6. 76 x 105

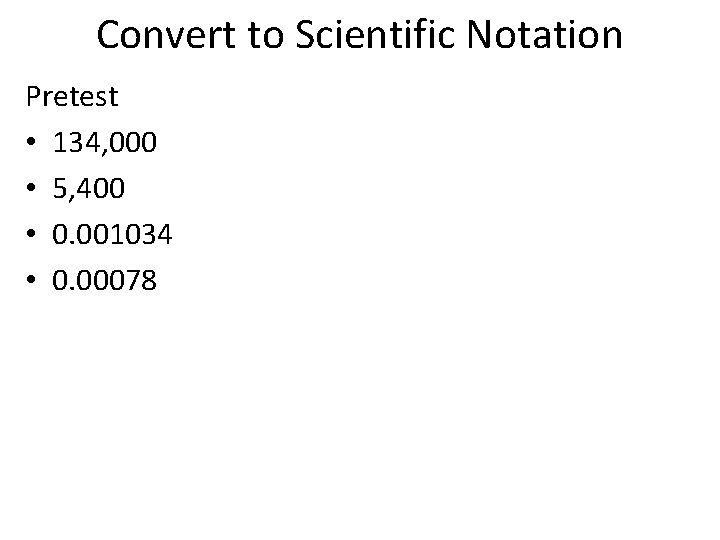
Convert to Scientific Notation Pretest • 134, 000 • 5, 400 • 0. 001034 • 0. 00078

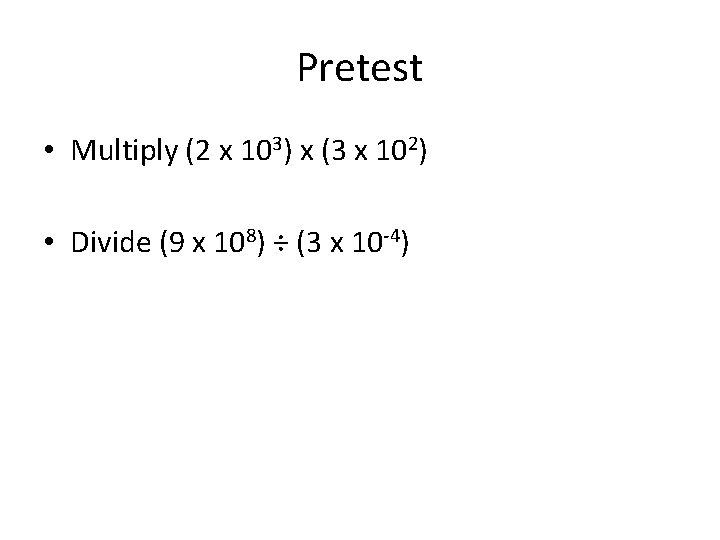
Pretest • Multiply (2 x 103) x (3 x 102) • Divide (9 x 108) ÷ (3 x 10 -4)

Multiplying / Dividing Multiplying Rules: – 1 st… multiply the numbers – 2 nd… add the exponents Dividing Rules – 1 st… divide the numbers – 2 nd… subtract the exponents

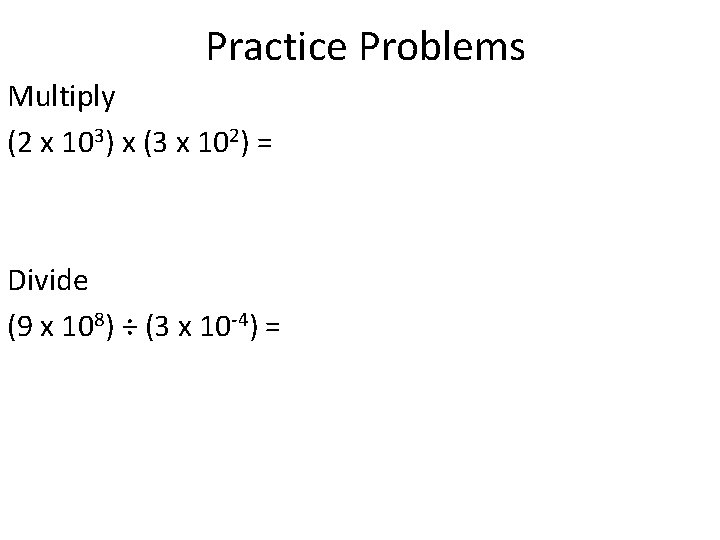
Practice Problems Multiply (2 x 103) x (3 x 102) = Divide (9 x 108) ÷ (3 x 10 -4) =

Rules: Conversions 1 st… write down what you know 2 nd… write down what you want to know 3 rd… what conversion factor are you going to use to get there? Convert 48 km to meters (factor: 1 km=1000 meters) or =______m 48000

Types of Measurements • Precise vs. Accurate

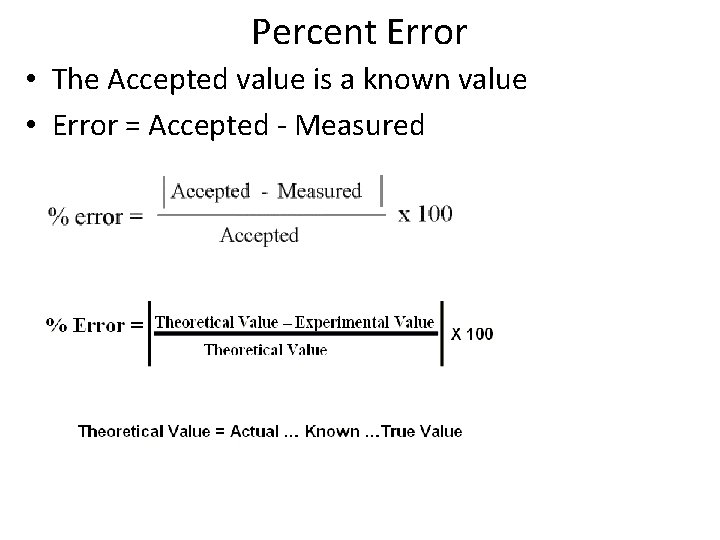
Percent Error • The Accepted value is a known value • Error = Accepted - Measured

% error example • The accepted density for copper is 8. 96 g/m. L. Calculate the percent error for each of these measurements. 1. 11% • 8. 86 g/m. L. 45% • 8. 92 g/m. L • 9. 00 g/m. L. 45% • 8. 98 g/m. L. 22%

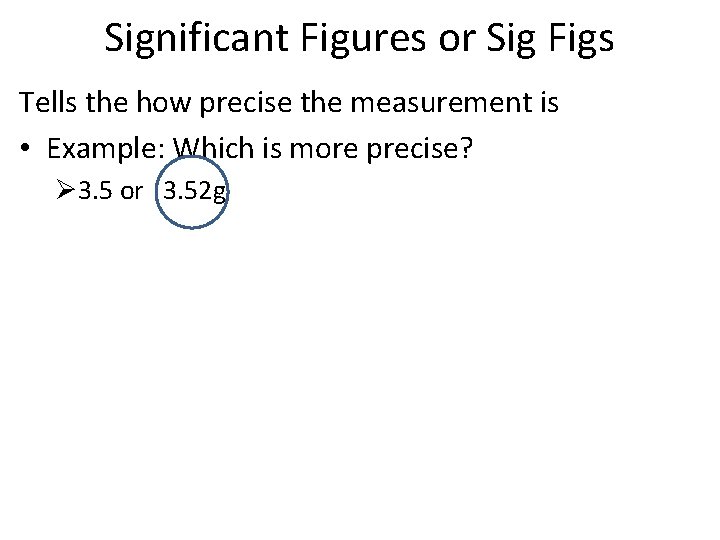
Significant Figures or Sig Figs Tells the how precise the measurement is • Example: Which is more precise? Ø 3. 5 or 3. 52 g

Rules for Sig Figs – pg. 39 1. Non-zero umbers are always significant 2. Zeros between non-zero numbers are always significant 3. All final zeros to the right of the decimal place are significant. 4. Zeros that act as placeholders are NOT significant. Convert quantities to scientific notation to remove the placeholders. 5. Counting numbers and defined constants have an infinite number of significant figures.

Examples – use your rules • Which numbers are significant? Ø 72. 3 Ø 60. 5 Ø 6. 20 Ø 0. 0253 Ø 4320 Ø 125000 • Help yourself out – convert to Scientific Notation