Crystal Growth GLY 4200 Fall 2012 1 Mineral

















- Slides: 21

Crystal Growth GLY 4200 Fall, 2012 1

Mineral Size • Mineral size - nm’s to tens of meters • Mineral mass nanograms to megagrams • Stibnite crystals 2

Methods of Crystal Growth • From solution, usually aqueous • From a melt • By sublimation from a gas phase 3

Nucleation • Usually form from the initial crystallization products of solutions or melts • Various ions must combine to form an initial regular structure pattern of a crystal • Usually requires supersaturation 4

Supersaturation • Achieved by: § Increasing concentration § Changing temperature § Changing pressure § Rate of change is important § Slow cooling leads to a few nuclei and large crystals § Rapid cooling leads to many nuclei, small crystals 5

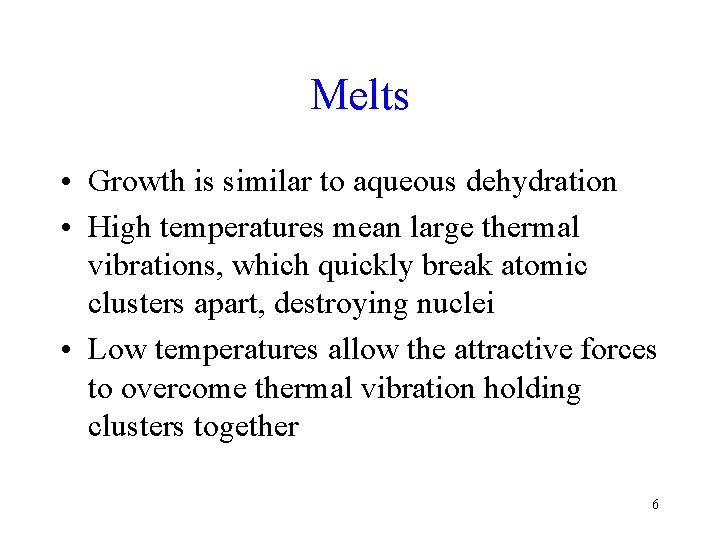
Melts • Growth is similar to aqueous dehydration • High temperatures mean large thermal vibrations, which quickly break atomic clusters apart, destroying nuclei • Low temperatures allow the attractive forces to overcome thermal vibration holding clusters together 6

Growth From Melt 7

Vapor • Cooling allows dissociated atoms or molecules to join • Examples: § Formation of snowflakes § Growth of ice on a window § Formation of sulfur crystals around fumaroles 8

Destruction of Nuclei • Nuclei have very large surface area/volume • Unsatisfied bonding on outer surfaces leads to dissolution • Crystallization only takes place when some nuclei survive long enough for growth to occur 9

Critical Size • If nuclei grow rapidly, their surface area/volume declines, and they may reach and exceed a critical size • Above the critical size, the nuclei are relatively stable, and growth can begin 10

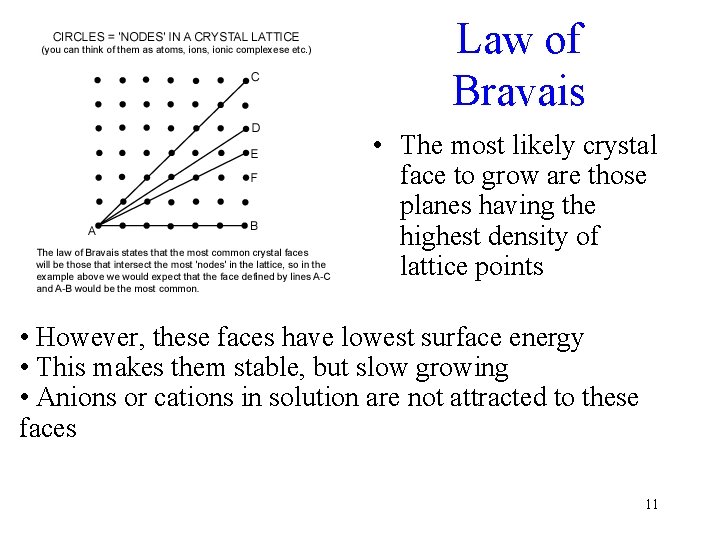
Law of Bravais • The most likely crystal face to grow are those planes having the highest density of lattice points • However, these faces have lowest surface energy • This makes them stable, but slow growing • Anions or cations in solution are not attracted to these faces 11

Rate of Growth • Faces composed of all anions or all cations are very high energy • They attract ions of the opposite sign, and grow rapidly • Eventually they grow themselves out of existence, leaving the slower growing faces 12

Vectorial Properties • Some properties of crystals depend on the direction in which they are measured • These are called vectorial properties • Examples: Hardness, electrical and thermal conductivity, speed of light, speed of seismic waves, thermal expansion, solution rate, and diffraction of X-rays 13

Variation of Vectorial Properties • Many vectorial properties vary discontinuously as direction is changed • Values of these properties pertain to a given crystallographic direction • Values of the property in crystallographic directions intermediate to two given directions do not very smoothly as the direction is changed 14

Discontinuous Vectorial Properties Examples • • • Color banding in minerals Dendritic growth Rate of solution etching by a solvent Cleavage Hardness 15

Color Bands • Tourmaline often shows color banding 16

Dendritic Mineral Habit • Dendritic formation of bright native silver crystals. • State of Maine Mine, Tombstone District, Cochise Co. , Arizona, USA 17

Continuous Vectorial Properties Examples • Index of refraction, related to the velocity of light • Seismic velocities in crystals • Electrical and thermal conductivity • Thermal expansivity 18

Crystal Intergrowths • During crystal growth, one crystalline substance may grow on a crystalline substance of different composition and structure • Such growths are known as epitaxial growths 19

Epitaxial Overgrowth Examples • The (010) plane of staurolite has a structure similar to kyanite § Kyanite’s (100) may epitaxially overgrow staurolite • Similarly, plagioclase sometimes overgrows microcline. 20

Epitaxis Photo • Epitaxial overgrowth of quartz on epidote • Green Monster Mine, Prince of Wales Island, Alaska 21
 Gly
Gly Pt(nh3)2cl2 isomers
Pt(nh3)2cl2 isomers Gly
Gly Dome crystal form
Dome crystal form Mineral crystal systems
Mineral crystal systems 9300 to 34800
9300 to 34800 Issai 4200
Issai 4200 Juniper
Juniper Issai 4000
Issai 4000 Checkpoint 12600
Checkpoint 12600 Step growth polymerization vs chain growth
Step growth polymerization vs chain growth Organic vs inorganic growth
Organic vs inorganic growth Primary growth and secondary growth in plants
Primary growth and secondary growth in plants Relative growth rates
Relative growth rates Geometric growth population
Geometric growth population Primary growth and secondary growth in plants
Primary growth and secondary growth in plants Pith
Pith Neoclassical growth theory vs. endogenous growth theory
Neoclassical growth theory vs. endogenous growth theory Luster mineral
Luster mineral Mineral identification dichotomous key
Mineral identification dichotomous key Kegunaan sumber mineral
Kegunaan sumber mineral Mga likas na yaman hilagang asya
Mga likas na yaman hilagang asya