COVALENT NETWORKS GIANT MOLECULES MACROMOLECULES COVALENT NETWORKS GIANT

- Slides: 7

COVALENT NETWORKS GIANT MOLECULES MACROMOLECULES

COVALENT NETWORKS GIANT MOLECULES MACROMOLECULES They all mean the same!

GIANT (MACRO) MOLECULES DIAMOND, GRAPHITE and SILICA Many atoms joined together in a regular array by a large number of covalent bonds GENERAL PROPERTIES MELTING POINT Very high structures are made up of a large number of covalent bonds, all of which need to be broken if the atoms are to be separated. ELECTRICAL Don’t conduct electricity - have no mobile ions or electrons but. . . Graphite conducts electricity STRENGTH Hard - exists in a rigid tetrahedral structure Diamond and silica (Si. O 2). . . but Graphite is soft

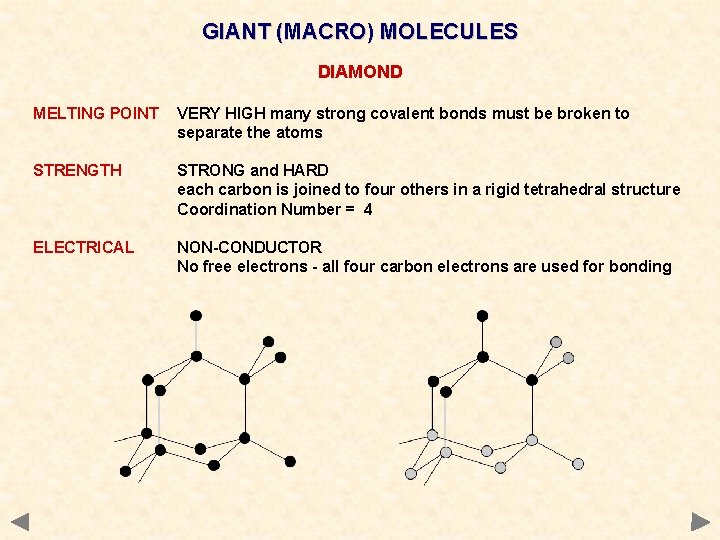
GIANT (MACRO) MOLECULES DIAMOND MELTING POINT VERY HIGH many strong covalent bonds must be broken to separate the atoms STRENGTH STRONG and HARD each carbon is joined to four others in a rigid tetrahedral structure Coordination Number = 4 ELECTRICAL NON-CONDUCTOR No free electrons - all four carbon electrons are used for bonding

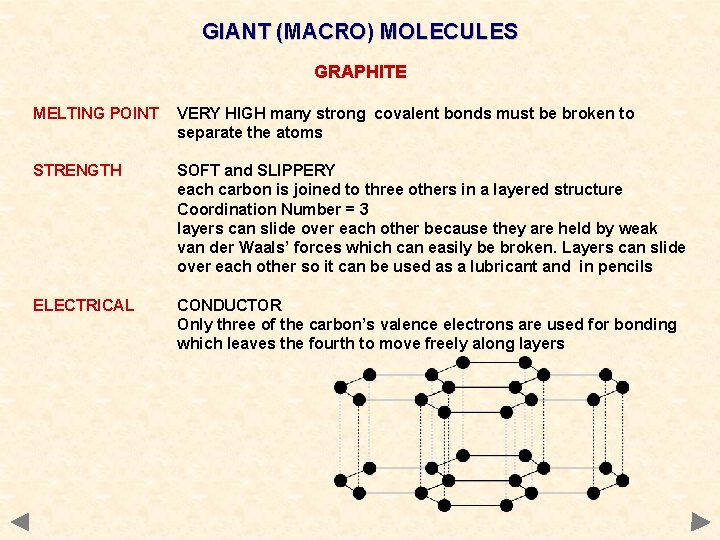
GIANT (MACRO) MOLECULES GRAPHITE MELTING POINT VERY HIGH many strong covalent bonds must be broken to separate the atoms STRENGTH SOFT and SLIPPERY each carbon is joined to three others in a layered structure Coordination Number = 3 layers can slide over each other because they are held by weak van der Waals’ forces which can easily be broken. Layers can slide over each other so it can be used as a lubricant and in pencils ELECTRICAL CONDUCTOR Only three of the carbon’s valence electrons are used for bonding which leaves the fourth to move freely along layers

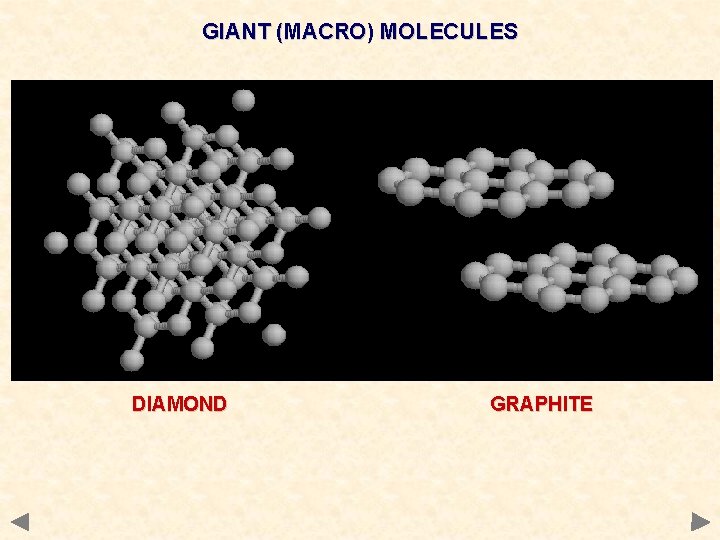
GIANT (MACRO) MOLECULES DIAMOND GRAPHITE

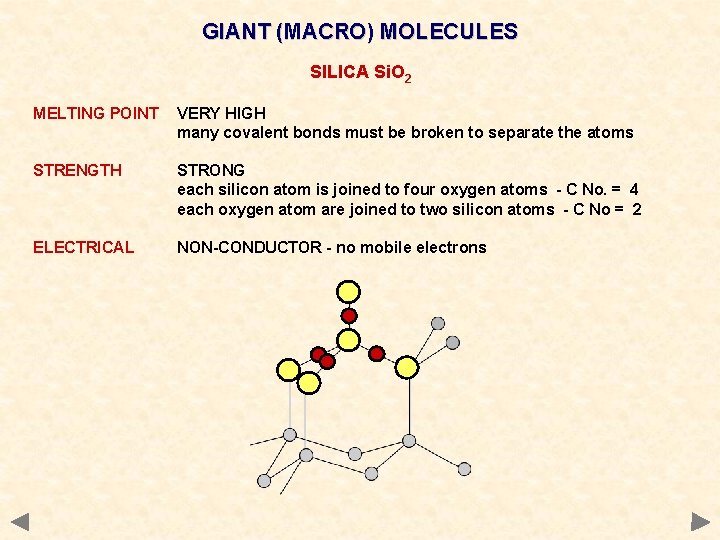
GIANT (MACRO) MOLECULES SILICA Si. O 2 MELTING POINT VERY HIGH many covalent bonds must be broken to separate the atoms STRENGTH STRONG each silicon atom is joined to four oxygen atoms - C No. = 4 each oxygen atom are joined to two silicon atoms - C No = 2 ELECTRICAL NON-CONDUCTOR - no mobile electrons