Course Title Nuclear Physics Course Code EEE202 LectureII

- Slides: 10

Course Title: Nuclear Physics Course Code: EEE-202 Lecture-II Shekh Md Mahmudul Islam, Lecturer, Department of Electrical and Electronic Engineering(EEE), University of Dhaka. Cell: +88 -01818402137 Email: mahmud@du. ac. bd ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 1

Last class I have discussed q Binding Energy q Mass Defect ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 2

Mathematical Problems: § Find the Binding Energy in Me. V of ordinary He for which M=4. 002603? § How much energy in Me. V required to completely dissociate U-235 Nucleus(atomic mass ) into its components protons and neutrons? § Calculate the average binding energy per mole of a U-235 isotope. Show your answer in KJ/mole? § Cl-35 has an exact mass of 34. 9689 amu. Calculate the mass defect of Cl-35? [Ans: 0. 321 amu] § Calculate the nuclear binding energy of Cu-63 which has a mass defect of 0. 594 amu? Express your answer in terms of J/mole? ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 3

Mathematical Problem q. Calculate the nuclear binding energy of a sulfur-32 atom. The measured atomic mass of a sulfur-32 is 31. 972 070 amu. q. Find mass defect: q. Convert amu to kg; 1 amu=1. 6605 *10 -27 kg ANSWER: 4. 36*10 -11 J. ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 4

Stable VS Unstable Nucleus q. In some atoms, the binding energy is great enough to hold the nucleus together. The nucleus of this kind of atom is said to be stable. q. In some atoms the binding energy is not strong enough to hold the nucleus together, and the nuclei of these atoms are said to be unstable. q. Unstable atoms will lose neutrons and protons as they attempt to become stable. q. Unstable atom radioactive atom ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 5

Stable VS Unstable Nucleus • • Stable nucleus – non-radioactive Unstable nucleus – radioactive less stable means more radioactive more stable means less radioactive. Q: What makes the nucleus a stable one? • It appears that neutron to proton (n/p) ratio is the dominant factor in nuclear stability. • This ratio is close to 1 for atoms of elements with low atomic number and increases as the atomic number increases. ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 6

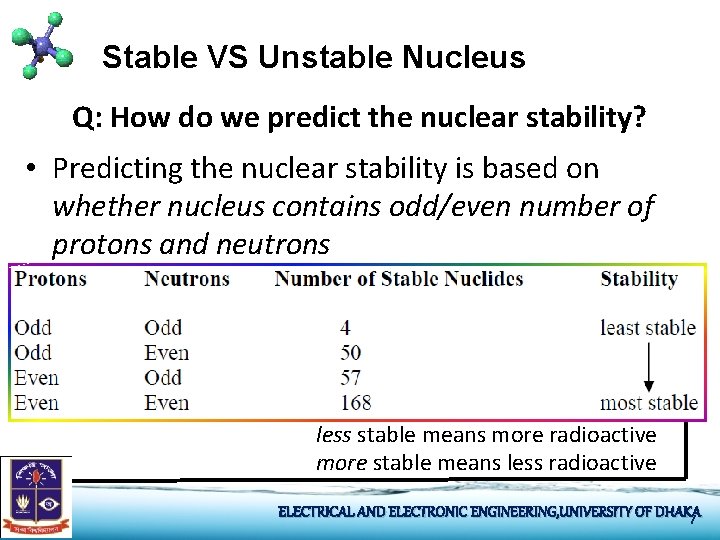
Stable VS Unstable Nucleus Q: How do we predict the nuclear stability? • Predicting the nuclear stability is based on whether nucleus contains odd/even number of protons and neutrons less stable means more radioactive more stable means less radioactive ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 7

Stable VS Unstable Nucleus Q: Based on the even-odd rule, predict which one would you expect to be radioactive in each pair? q 16/8 O and 17/8 O q 35/17 Cl and 36/17 Cl q 20/10 Ne and 17/10 Ne q 40/20 Ca and 45/20 Ca q 195/80 Hg and 196/80 Hg ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 8

Stable VS Unstable Nucleus Q: Based on the even-odd rule, predict which one would you expect to be radioactive in each pair? q 16/8 O and 17/8 O q 35/17 Cl and 36/17 Cl q 20/10 Ne and 17/10 Ne q 40/20 Ca and 45/20 Ca q 195/80 Hg and 196/80 Hg ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 9

Stable VS Unstable Nucleus q The 16/8 O contains 8 protons and 8 neutrons (even-even) and the 17/8 O contains 8 protons and 9 neutrons (even-odd). Therefore, 17/8 O is radioactive. q The 35/17 Cl has 17 protons and 18 neutrons (odd-even) and the 36/17 Cl has 17 protons and 19 neutrons (odd-odd). Hence, 36/17 Cl is radioactive. q The 20/10 Ne contains 10 protons and 10 neutrons (even-even) and the 17/10 Ne contains 10 protons and 7 neutrons (even-odd). Therefore, 17/10 Ne is radioactive. q The 40/20 Ca has even-even situation and 45/20 Ca has even-odd situation. Thus, 45/20 Ca is radioactive. q The 195/80 Hg has even number of protons and odd number of neutrons and the 196/80 Hg has even number of protons and even number of neutrons. Therefore, 195/80 Hg is radioactive. ELECTRICAL AND ELECTRONIC ENGINEERING, UNIVERSITY OF DHAKA 1 0