Compliance with international guidelines on investigation of testicular






- Slides: 15

Compliance with international guidelines on investigation of testicular tumours ASHLEY FERRO IQBAL MIAKHIL Project Registration Number - 2178 Date of presentation: 28 th November 2018 Start Date 23 Jul 18 / End Date 28 Nov 18 Surgical Division / Department of Urology

Name Status in team Ashley Ferro Project Lead clinician Iqbal Miakhil Clinical Audit Lead / Supervisor Audit team Department

Background - Testicular Cancer Demographics • 1% male tumours in the UK • lifetime risk 1: 500 with normally descended testes; incidence rising ? why? • peak incidence – 30 -40 years (Seminoma) • highest risk in Caucasian populations

Risk factors • Caucasian • undescended tests: • unilateral – 4 x risk • bilateral – 10 x risk • contralateral GCT • subfertility – 2 x risk • kleinfelters • kallmann • prenatal oestrogen exposure

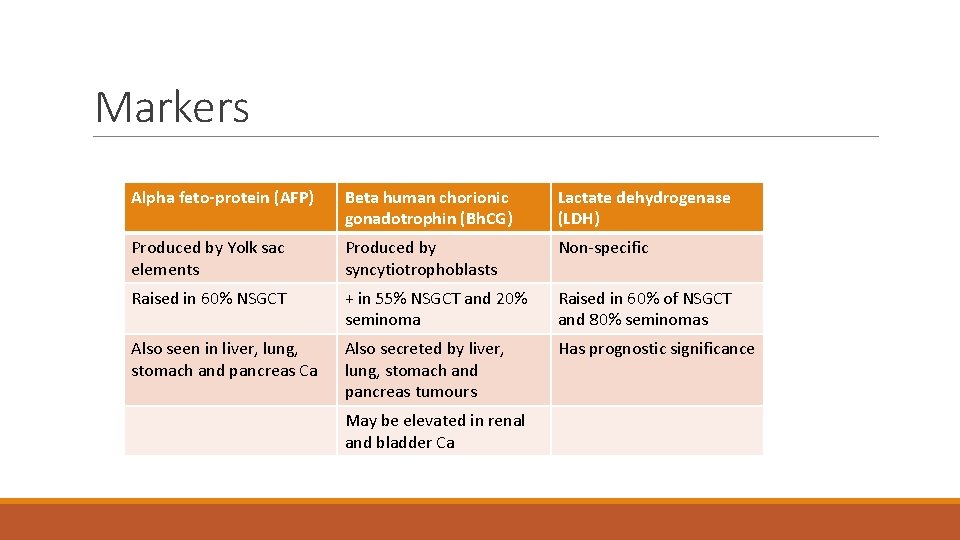
Markers Alpha feto-protein (AFP) Beta human chorionic gonadotrophin (Bh. CG) Lactate dehydrogenase (LDH) Produced by Yolk sac elements Produced by syncytiotrophoblasts Non-specific Raised in 60% NSGCT + in 55% NSGCT and 20% seminoma Raised in 60% of NSGCT and 80% seminomas Also seen in liver, lung, stomach and pancreas Ca Also secreted by liver, lung, stomach and pancreas tumours Has prognostic significance May be elevated in renal and bladder Ca

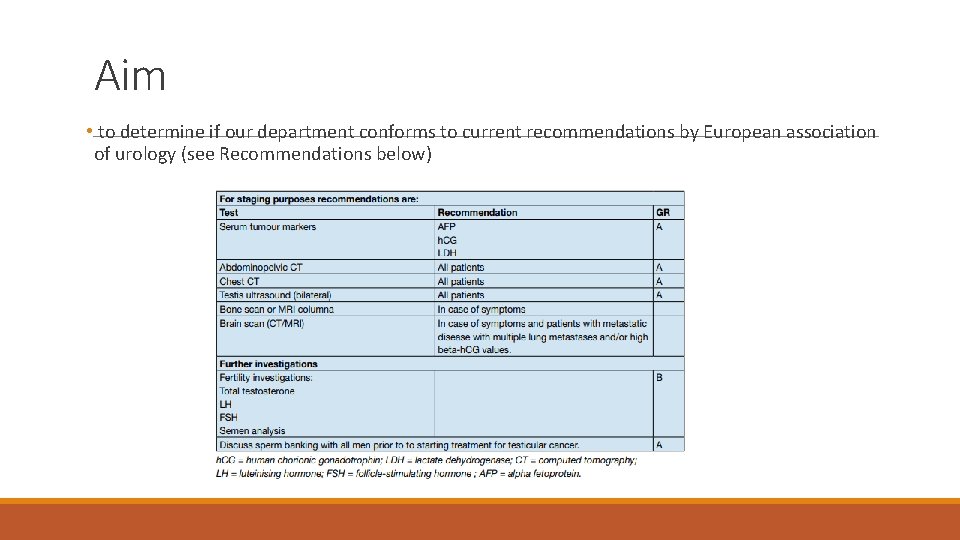
Aim • to determine if our department conforms to current recommendations by European association of urology (see Recommendations below)

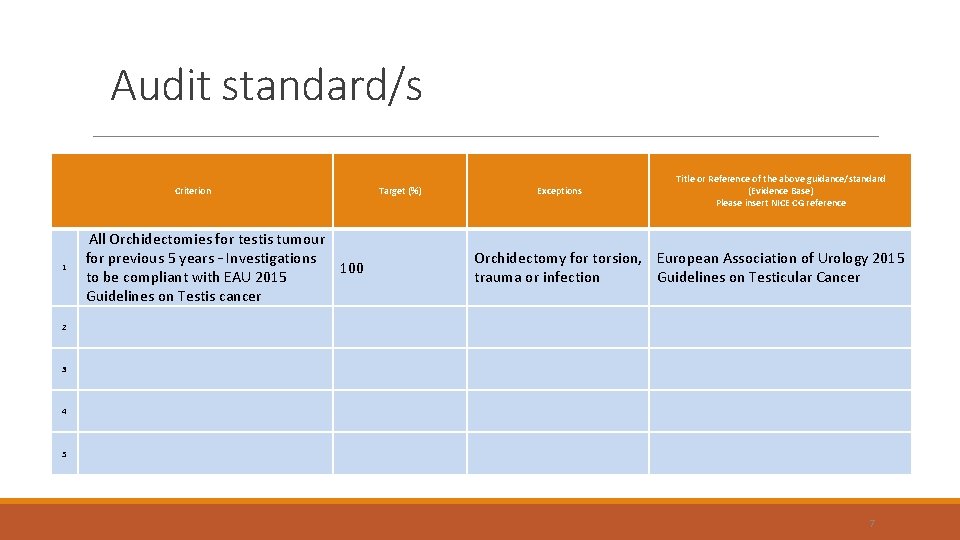
Audit standard/s Criterion 1 2 3 4 5 All Orchidectomies for testis tumour for previous 5 years – Investigations 100 to be compliant with EAU 2015 Guidelines on Testis cancer Target (%) Exceptions Title or Reference of the above guidance/standard (Evidence Base) Please insert NICE CG reference Orchidectomy for torsion, European Association of Urology 2015 trauma or infection Guidelines on Testicular Cancer 7

Methods § All patients with ICD code for ‘orchidectomy’ were identified between April 2013 to present §orchidectomies indicated for torsion, trauma or epididymitis were excluded §data was extracted into a data collection form and the following parameters recorded: §age §admitting complaint (as per US request) §affected side §US-reported size of mass §US description §presence/absence of Staging CTAP §suspicion of nodal metastasis? §concern for metastasis? §actual tumour size (from histology) §tumour classification §immunostaining §TNM §Bh. CG §AFP §LDH

Summary 168 orchidectomies 100 orchidectomies indicated for mass 78 histologically -confirmed malignancies

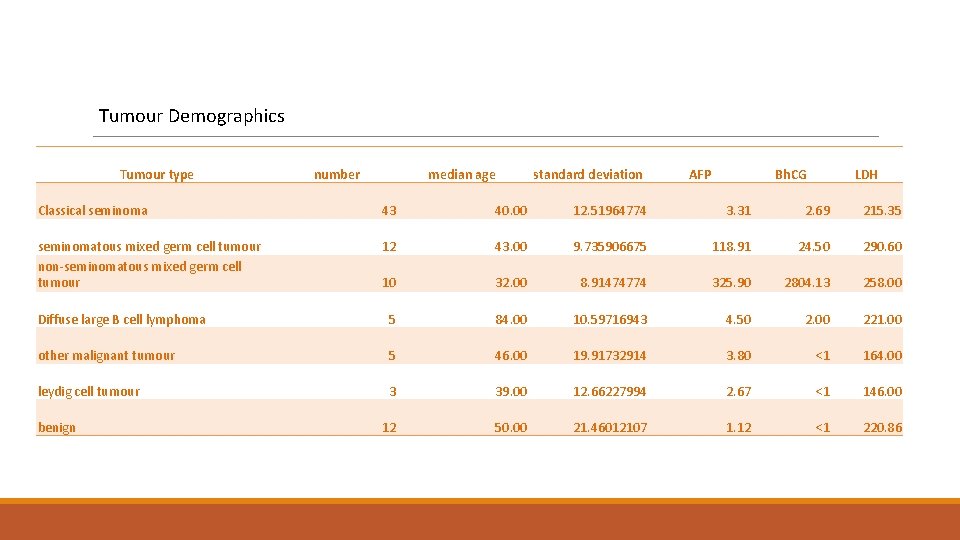
Tumour Demographics Tumour type number median age standard deviation AFP Bh. CG LDH Classical seminoma 43 40. 00 12. 51964774 3. 31 2. 69 215. 35 seminomatous mixed germ cell tumour non-seminomatous mixed germ cell tumour 12 43. 00 9. 735906675 118. 91 24. 50 290. 60 10 32. 00 8. 91474774 325. 90 2804. 13 258. 00 Diffuse large B cell lymphoma 5 84. 00 10. 59716943 4. 50 2. 00 221. 00 other malignant tumour 5 46. 00 19. 91732914 3. 80 <1 164. 00 leydig cell tumour 3 39. 00 12. 66227994 2. 67 <1 146. 00 12 50. 00 21. 46012107 1. 12 <1 220. 86 benign

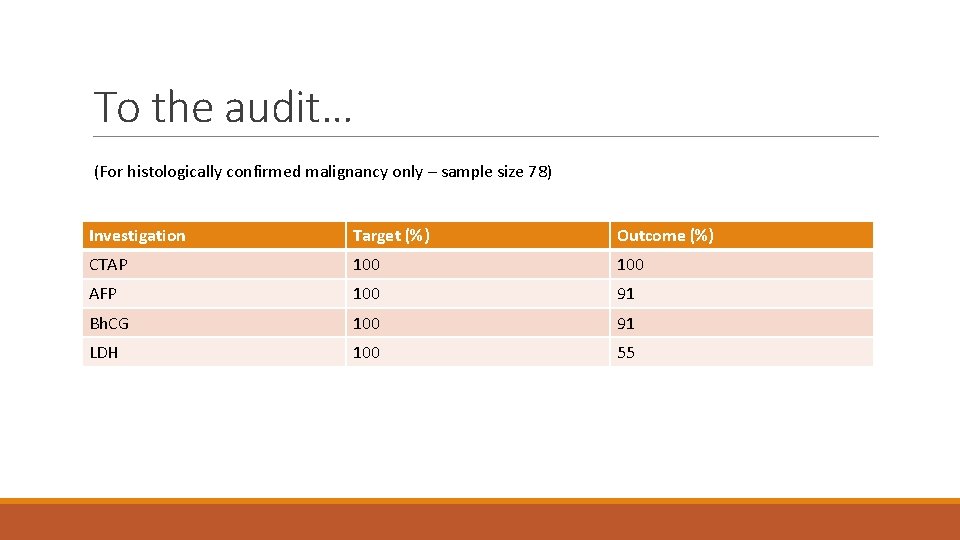
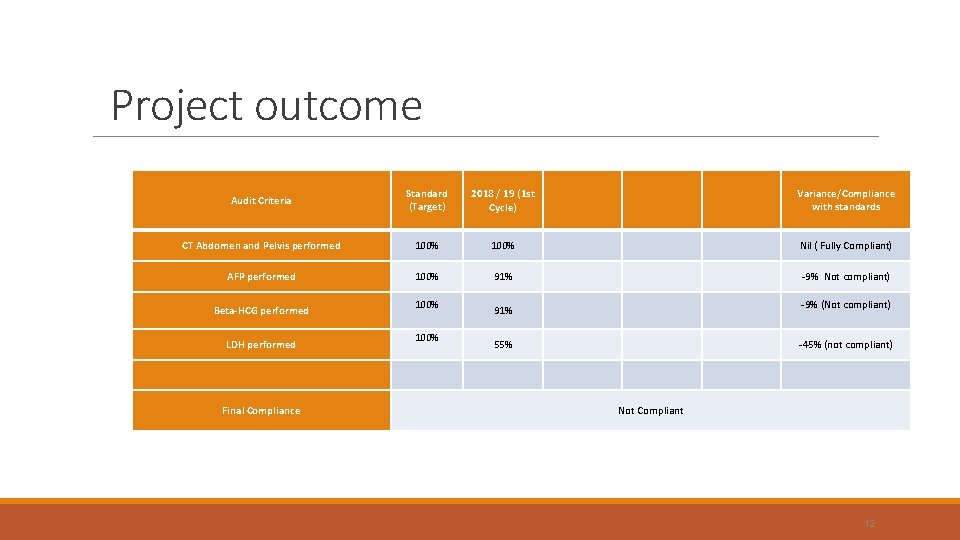
To the audit… (For histologically confirmed malignancy only – sample size 78) Investigation Target (%) Outcome (%) CTAP 100 AFP 100 91 Bh. CG 100 91 LDH 100 55

Project outcome Audit Criteria Standard (Target) 2018 / 19 (1 st Cycle) Variance/Compliance with standards CT Abdomen and Pelvis performed 100% Nil ( Fully Compliant) AFP performed 100% 91% -9% Not compliant) Beta-HCG performed LDH performed Final Compliance 100% -9% (Not compliant) 91% 55% -45% (not compliant) Not Compliant 12

Recommendations and Suggestions for improvement 1. Discussed at Urology CG ½ Day 2. “Suspected testicular tumour investigation panel” on ICE would signficantly improve compliance with the Audit Parameters 1. 2. 3. 4. 5. US scrotum AFP LDH Bh. CG CTAP

SMART Outcomes 14

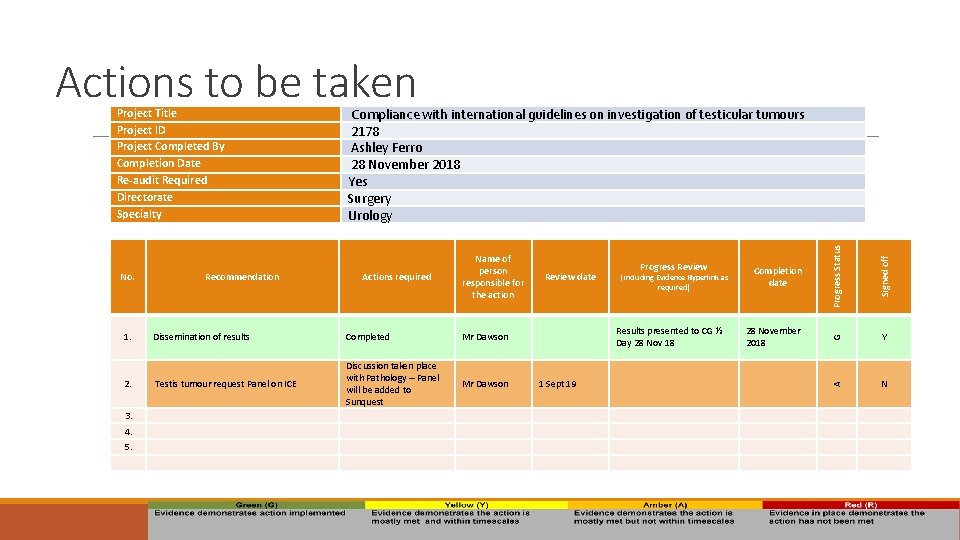
Actions to be taken 4. 1 Sept 19 Signed off Mr Dawson Progress Status 3. 28 November 2018 G Testis tumour request Panel on ICE Results presented to CG ½ Day 28 Nov 18 Mr Dawson Completion date ( including Evidence Hyperlink as required) Y A 2. Discussion taken place with Pathology – Panel will be added to Sunquest 5. Completed Progress Review date N Dissemination of results Actions required 1. Recommendation Name of person responsible for the action No. Compliance with international guidelines on investigation of testicular tumours 2178 Ashley Ferro 28 November 2018 Yes Surgery Urology Project Title Project ID Project Completed By Completion Date Re-audit Required Directorate Specialty 15
 Legal regulations compliance and investigation
Legal regulations compliance and investigation Cremasteric reflex nerve
Cremasteric reflex nerve Inflammation
Inflammation Christina shareef
Christina shareef Teratoma
Teratoma Testicular detorsion maneuver
Testicular detorsion maneuver Neoplasia testicular
Neoplasia testicular Normal testicle size for age
Normal testicle size for age Testicular function
Testicular function Epigenital tubules
Epigenital tubules Testicular feminization syndrome
Testicular feminization syndrome Lobes of prostate
Lobes of prostate Rplnd testicular cancer
Rplnd testicular cancer Diaphrosis
Diaphrosis Parenchimul testicular
Parenchimul testicular Strength of materials pdf
Strength of materials pdf