Collection of Survival and Clinical Data for Multicenter








- Slides: 25

Collection of Survival and Clinical Data for Multicenter Clinical Trials Using the Qlikview App Provided By CIBMTR 02/20/2019 1: 55 PM

Presentation Anderson João Simione Data Analyst Hospital Amaral Carvalho Cinthya Corrêa da Silva Biomedical / Data Manager Hospital Israelita Albert Einstein There are no conflicts of interest to disclose

Objective To evaluate Overall Survival (OS) and clinical characteristics of patients undergoing hematopoietic stem cell transplantation (HSCT) in Brazil while testing the usefulness and efficacy of the Qlikview app.

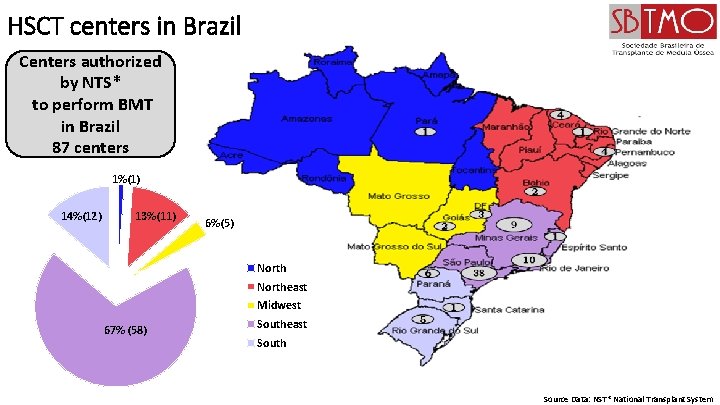
HSCT centers in Brazil Centers authorized by NTS* to perform BMT in Brazil 87 centers 1%(1) 14%(12) 13%(11) 6%(5) Northeast Midwest 67% (58) Southeast South Source Data: NST* National Transplant System

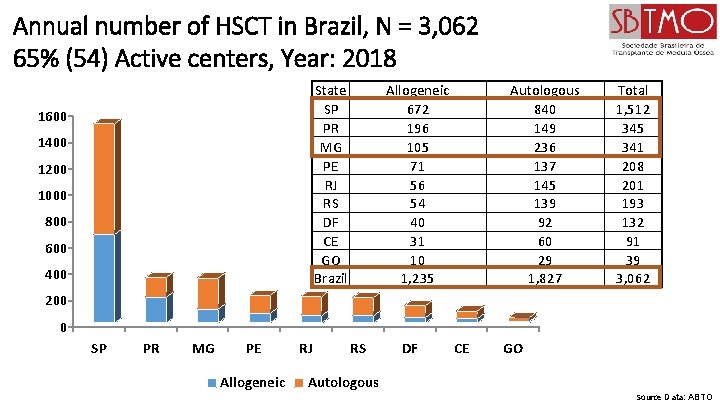
Annual number of HSCT in Brazil, N = 3, 062 65% (54) Active centers, Year: 2018 State SP PR MG PE RJ RS DF CE GO Brazil 1600 1400 1200 1000 800 600 400 Allogeneic 672 196 105 71 56 54 40 31 10 1, 235 Autologous 840 149 236 137 145 139 92 60 29 1, 827 Total 1, 512 345 341 208 201 193 132 91 39 3, 062 200 0 SP PR MG PE Allogeneic RJ RS DF CE GO Autologous Source Data: ABTO

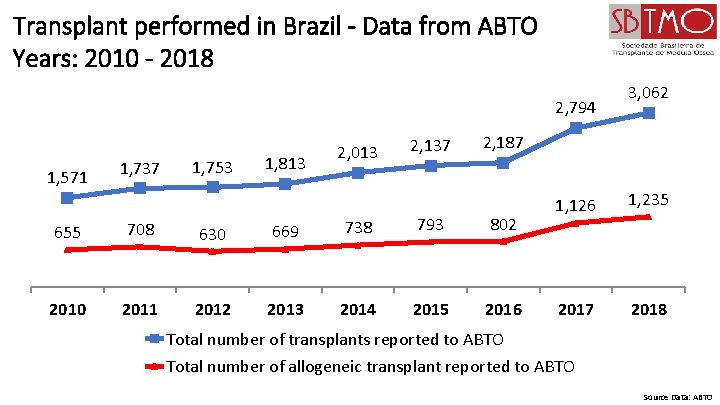
Transplant performed in Brazil - Data from ABTO Years: 2010 - 2018 2, 794 1, 571 1, 737 1, 753 1, 813 2, 013 2, 137 3, 062 2, 187 655 708 630 669 738 793 802 2010 2011 2012 2013 2014 2015 2016 1, 126 1, 235 2017 2018 Total number of transplants reported to ABTO Total number of allogeneic transplant reported to ABTO Source Data: ABTO

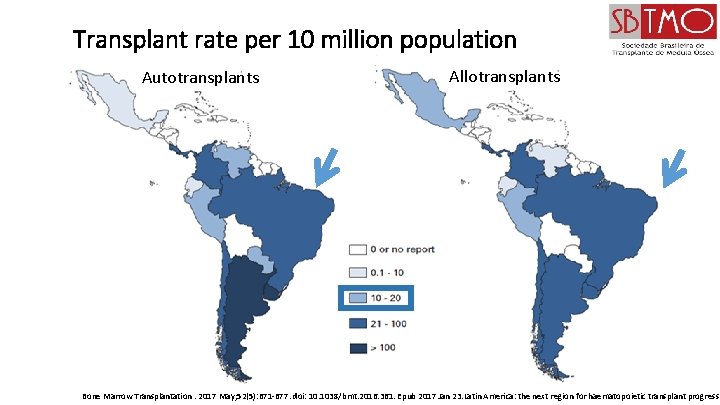
Transplant rate per 10 million population Autotransplants Allotransplants Bone Marrow Transplantation. 2017 May; 52(5): 671 -677. doi: 10. 1038/bmt. 2016. 361. Epub 2017 Jan 23. Latin America: the next region for haematopoietic transplant progress

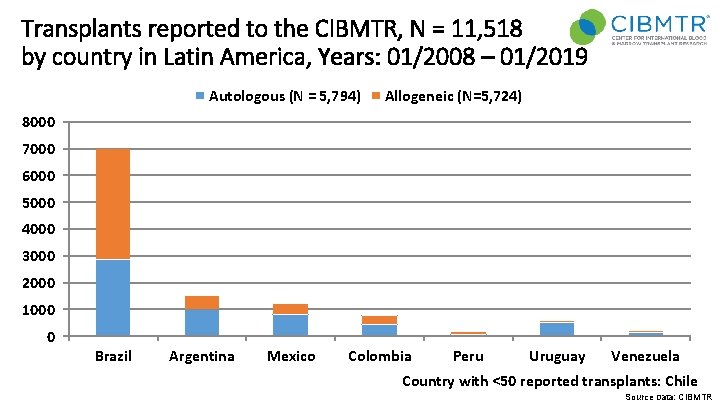
Transplants reported to the CIBMTR, N = 11, 518 by country in Latin America, Years: 01/2008 – 01/2019 Autologous (N = 5, 794) Allogeneic (N=5, 724) 8000 7000 6000 5000 4000 3000 2000 1000 0 Brazil Argentina Mexico Colombia Peru Uruguay Venezuela Country with <50 reported transplants: Chile Source Data: CIBMTR

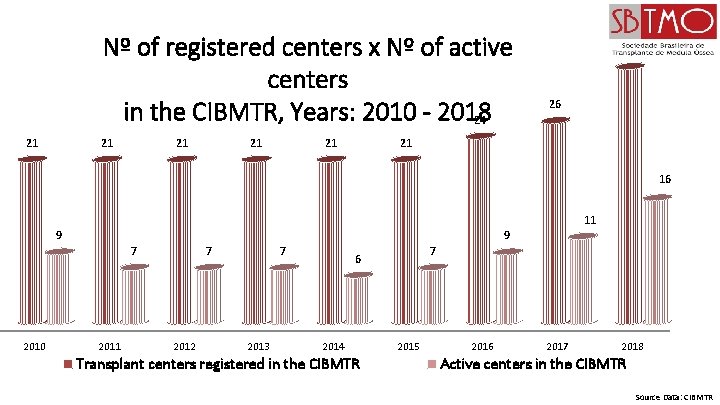
Nº of registered centers x Nº of active centers in the CIBMTR, Years: 2010 - 2018 24 21 21 21 33 26 21 16 11 9 9 7 2010 2011 7 2012 7 2013 7 6 2014 Transplant centers registered in the CIBMTR 2015 2016 2017 2018 Active centers in the CIBMTR Source Data: CIBMTR

Scientific Difficulties of Brazilian Centers 1 st - Brazil does not have a consolidated database so far; 2 st - Difficulty of analysis such as: demographic data, outcomes and scientific production (multicenter study); 3 st - Difficulty in developing scientific works;

Multicenter Curso: registry. Guia of autologous and allogeneic hematopoietic cell Transplante de Preenchimento de Dados Essencias Pré e Pós transplantations for malignant and non-malignant diseases performed in Brazil at the Center for International Blood and Marrow Transplant Research(CIBMTR) 1 st Development of a protocol in Brazil in order to formalize the registration of BMT data in the country to the CIBMTR; 2 st Multicentric study; 3 st Submission to local Ethics Committee in Research; 4 st Submission to Ethics National Commission in Research; 5 st 13 centers participating in the project.

Curso: Guia de Preenchimento de Dados Essencias Pré e Pós Transplante Some Participating Centers in this research

Step by Step 1 st Contact the participating centers of the study send the data to the center that is the principal investigator 2 st We guide participating centers on how to access and extract the data in the portal of the CIBMTR - e. DBt. C, through Qlik. View 3 st We developed the homemade tutorial to assist in extracting data in e. DBt. C: e. DBt. C_Tutorial. mp 4 4 st We send the video to data manager or professional responsible for processing it by Whatsapp

Step by Step 5 st step

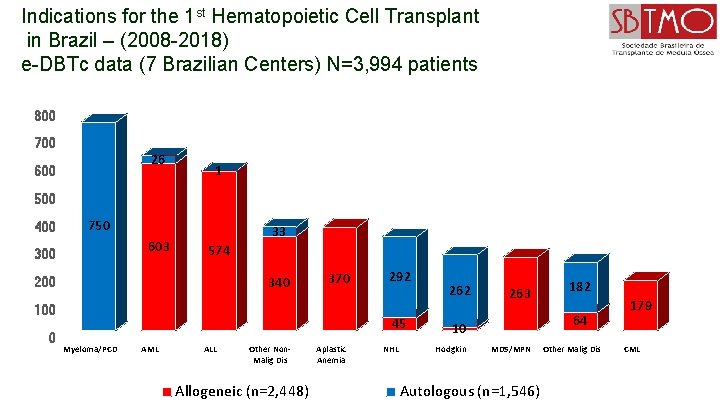
Indications for the 1 st Hematopoietic Cell Transplant in Brazil – (2008 -2018) e-DBTc data (7 Brazilian Centers) N=3, 994 patients 800 700 26 600 1 500 400 750 603 300 33 574 340 200 370 100 0 292 45 Myeloma/PCD AML ALL Other Non. Malig Dis Allogeneic (n=2, 448) Aplastic Anemia NHL 262 263 64 10 Hodgkin 182 MDS/MPN Autologous (n=1, 546) Other Malig Dis 179 CML

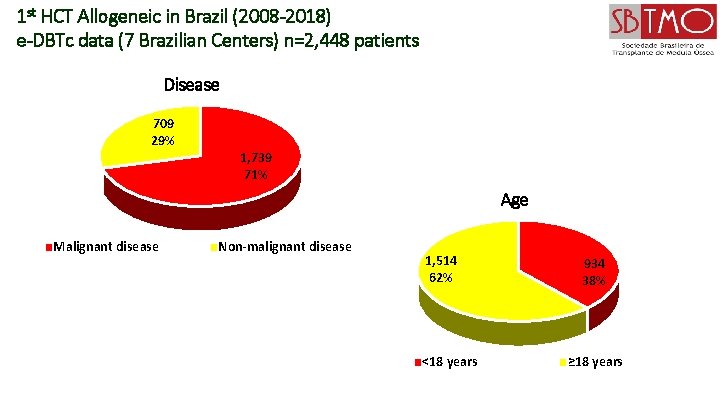
1 st HCT Allogeneic in Brazil (2008 -2018) e-DBTc data (7 Brazilian Centers) n=2, 448 patients Disease 709 29% 1, 739 71% Age Malignant disease Non-malignant disease 1, 514 62% <18 years 934 38% ≥ 18 years

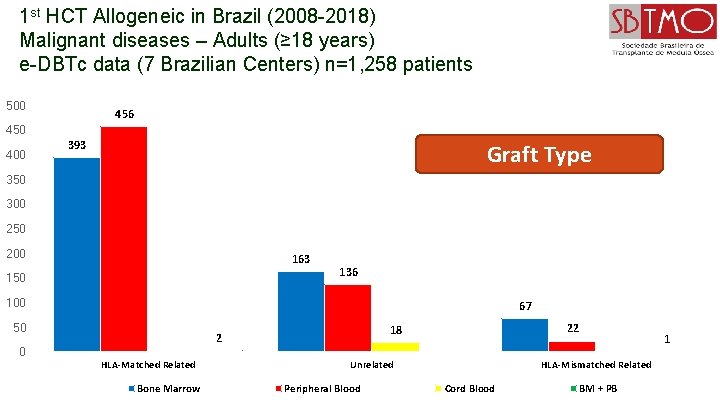
1 st HCT Allogeneic in Brazil (2008 -2018) Malignant diseases – Adults (≥ 18 years) e-DBTc data (7 Brazilian Centers) n=1, 258 patients 500 456 450 400 393 Graft Type 350 300 250 200 163 150 136 100 67 50 0 HLA-Matched Related Bone Marrow 22 18 2 Unrelated Peripheral Blood 1 HLA-Mismatched Related Cord Blood BM + PB

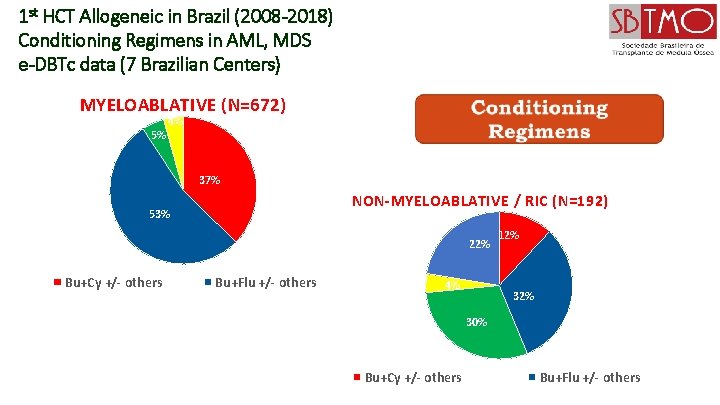
1 st HCT Allogeneic in Brazil (2008 -2018) Conditioning Regimens in AML, MDS e-DBTc data (7 Brazilian Centers) MYELOABLATIVE (N=672) 5% 4% 37% NON-MYELOABLATIVE / RIC (N=192) 53% 22% Bu+Cy +/- others Bu+Flu +/- others 4% 12% 30% Bu+Cy +/- others Bu+Flu +/- others

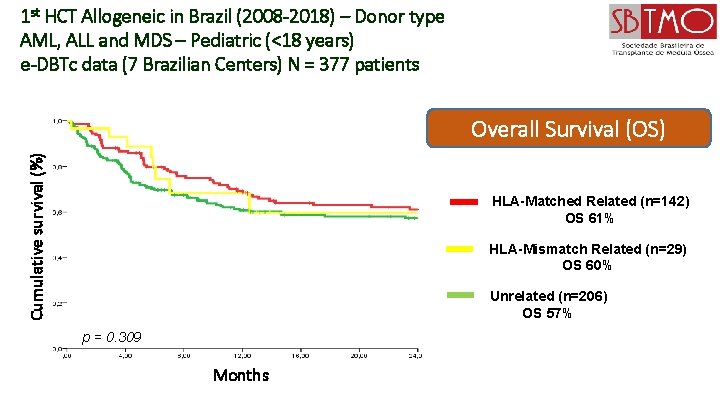
1 st HCT Allogeneic in Brazil (2008 -2018) – Donor type AML, ALL and MDS – Pediatric (<18 years) e-DBTc data (7 Brazilian Centers) N = 377 patients Cumulative survival (%) Overall Survival (OS) HLA-Matched Related (n=142) OS 61% HLA-Mismatch Related (n=29) OS 60% Unrelated (n=206) OS 57% p = 0. 309 Months

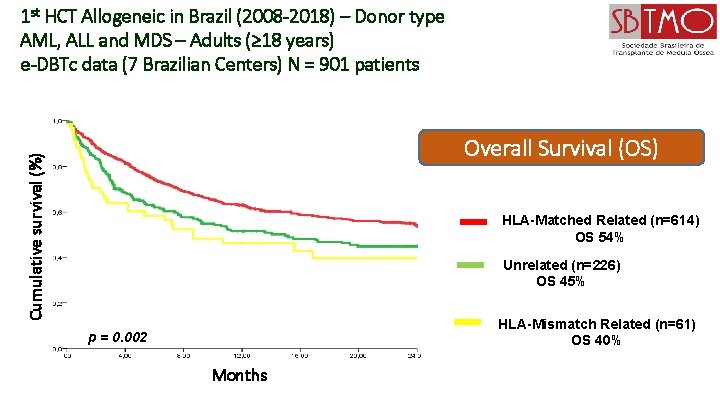
1 st HCT Allogeneic in Brazil (2008 -2018) – Donor type AML, ALL and MDS – Adults (≥ 18 years) e-DBTc data (7 Brazilian Centers) N = 901 patients Cumulative survival (%) Overall Survival (OS) HLA-Matched Related (n=614) OS 54% Unrelated (n=226) OS 45% HLA-Mismatch Related (n=61) OS 40% p = 0. 002 Months

Conclusion The Qlikview it is a useful tool for countries where a national database is not sufficient The Qlikview app allowed the standardized collection and analysis of data Several analysis can be created just with the data downloaded in the e-DBTc

Future Perspectives Data quality improvement Regular analyses the Brazilian data and share with the Brazilian Centers Increase of the number of the active centers in the CIBMTR

2018 Brazilian meeting - Data Manager

Participants Centers Nelson Hamerschlak , MD, Ph. D, Medical Coordinator blood and marrow transplantation service - HIAE , President of SBTMO • Cintia Monteiro – Clinical Nurse Specialist, Data manager – IOP / GRAAC • Cinthya Corrêa da Silva, Data manager blood and marrow transplantation service - HIAE • Victor Gottardello Zecchin - MD, MBA, Medical Coodinator Hemato ansd HSCT - IOP/ GRAACC • Anderson João Simioni, Data analyst blood and marrow transplantation service - HAC • Roberto Luiz da Silva , MD, Medical Coordinator – Hemato and HSCT – IBCC • eliz Regina das Neves, Data analyst/ Data manager blood and marrow transplantation service - UFPR • Bruna Letícia da Silva Santos Geraldo , Data manager, Nurse coordinator – Hemato and HSCT – Bio Sana’s • Iracema Esteves , MD, Ph. D, Hemato and HSCT - HIAE • • Vergilio Antônio Rensi Colturato vcolturato , Medical Coordinator blood and marrow transplantation service – HAC Fernando Barroso Duarte, MD, Ph. D. , Medical Coordinator - Hemato and HSCT – UFC • Germison Silva Lopes , MD, Hemato and HSCT - UFC • Afonso Celso Vigorito, MD, Ph. D, Hemocentro - UNICAMP • Marcos Paulo Colella, MD, MSc, Hemocentro -UNICAMP • Larissa Codogno Guzelotto, Data Manager, Nurse coordinator - Hemato and HCT – Hemocentro - UNICAMP • • • Carmem Maria Sales Bonfim , MD, Ph. D – Director pediatric blood and marrow transplantation service - UFPR Vaneuza Araújo Moreira Funke Md p, Assistant Professor of Hematology Federal University of Paraná , Head of Adult HSCT Center -Complexo Hospital De Clínicas, Federal University of Parana. Prof. Vaneuza Funke Hematologia - UFPR

 Multicenter study design
Multicenter study design State of survival survival of the fittest tweak
State of survival survival of the fittest tweak State of survival survival of the fittest stages
State of survival survival of the fittest stages Data collection procedure and data analysis
Data collection procedure and data analysis Landsat collection 1 vs collection 2
Landsat collection 1 vs collection 2 Documentary collection vs documentary credit
Documentary collection vs documentary credit Data collection secondary data sources
Data collection secondary data sources Data collection organization and presentation
Data collection organization and presentation Collection of interrelated data and programs
Collection of interrelated data and programs Collection and analysis of rate data
Collection and analysis of rate data Gis data input
Gis data input Data collection management and analysis
Data collection management and analysis Raw facts
Raw facts What is primary data.
What is primary data. Analysis and findings example
Analysis and findings example Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Typiska novell drag
Typiska novell drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Ekologiskt fotavtryck
Ekologiskt fotavtryck Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Tidbok yrkesförare
Tidbok yrkesförare Anatomi organ reproduksi
Anatomi organ reproduksi Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare