A Multicenter Randomized Clinical Trial to evaluate the




- Slides: 13

A Multicenter Randomized Clinical Trial to evaluate the Efficiency of small vessel coronary treatment using Paclitaxel drug-coated balloon vs. Zotarolimus-eluting stent: RAMSES trial Victor Alfonso Jimenez Diaz, MD, MPH Cardiology department, Hospital Alvaro Cunqueiro, University Hospital of Vigo, Spain On behalf of REAC TAVI study investigators

Victor A. Jimenez Diaz, MD, MPH I have no relevant financial relationships

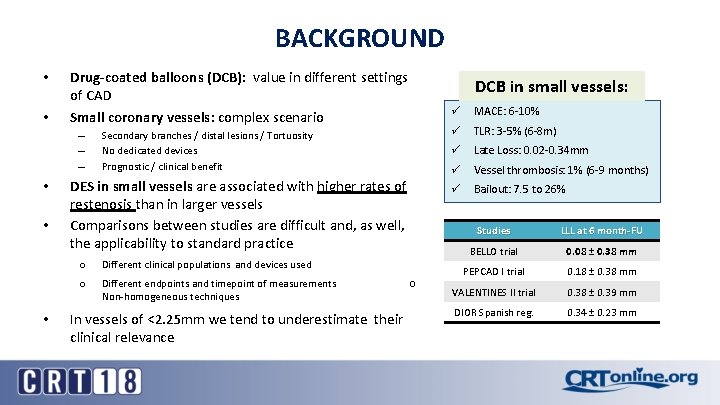
BACKGROUND • • Drug-coated balloons (DCB): value in different settings of CAD Small coronary vessels: complex scenario – – – • • • DCB in small vessels: Secondary branches / distal lesions / Tortuosity No dedicated devices Prognostic / clinical benefit DES in small vessels are associated with higher rates of restenosis than in larger vessels Comparisons between studies are difficult and, as well, the applicability to standard practice o Different clinical populations and devices used o Different endpoints and timepoint of measurements Non-homogeneous techniques In vessels of <2. 25 mm we tend to underestimate their clinical relevance ○ ü MACE: 6 -10% ü TLR: 3 -5% (6 -8 m) ü Late Loss: 0. 02 -0. 34 mm ü Vessel thrombosis: 1% (6 -9 months) ü Bailout: 7. 5 to 26% Studies LLL at 6 month-FU BELLO trial 0. 08 ± 0. 38 mm PEPCAD I trial 0. 18 ± 0. 38 mm VALENTINES II trial 0. 38 ± 0. 39 mm DIOR Spanish reg. 0. 34 ± 0. 23 mm

METHODS RAMSES trial: Paclitaxel DCB vs. Zotarolimus DES in small coronary vessels • • Prospective, multicenter, randomized controlled trial Patients with de novo coronary lesions located in small vessels – Reference vessel diameter: 2. 00 to 2. 75 mm • Randomized to: IN. PACT Falcon-DCB – Paclitaxel-DCB (IN. PACT Falcon, Medtronic Inc); or – Zotarolimus-DES (Resolute Integrity, Medtronic Inc) • Primary endpoint: – Composite of major adverse cardiac events ([MACEs] - cardiac death, MI, CABG, and clinically-driven TVR or CD-TLR at 12 month follow-up Resolute Integrity-DES

METHODS RAMSES trial: Paclitaxel DCB vs. Zotarolimus DES in small coronary vessels • Initiated Investigator Study • Executive and Clinical Events Committee (5 members) • All patients/events monitorized by an independent CRO • 7 centers in Spain: – H. Álvaro Cunqueiro, Vigo: Andrés Íñiguez – H. Universitario Ciudad Real: Fernando Lozano – H. Universitario de Canarias: Francisco Bosa – H. Carlos Haya: José Urbano – H. Universitario León: Felipe Fernández – H. Virgen de la Victoria: José María Hernández – H. Virgen de las Nieves: Rafael Melgares IN. PACT Falcon-DCB Resolute Integrity-DES

METHODS RAMSES trial: Paclitaxel DCB vs. Zotarolimus DES in small coronary vessels • Inclusion criteria • Exclusion criteria • CAD with de novo lesions in native coronary • Left main disease, CTOs, Bifurcations arteries • Severely calcified lesions, ISR Stenosis severity of ≥ 70% by visual stimation or • Lesions in LIMA, RIMA or venous bypasess >50% stenosis by QCA • AMI in the 48 hr pre-PCI Coronary vessels diameter between 2, 00 and • Severe renal failure 2, 75 mm • Allergy or contraindications to ASA or P 2 Y 12 inhibitors • Allergy to contrast agents • Life expectancy <1 year • • • Lesion length of ≤ 25 mm

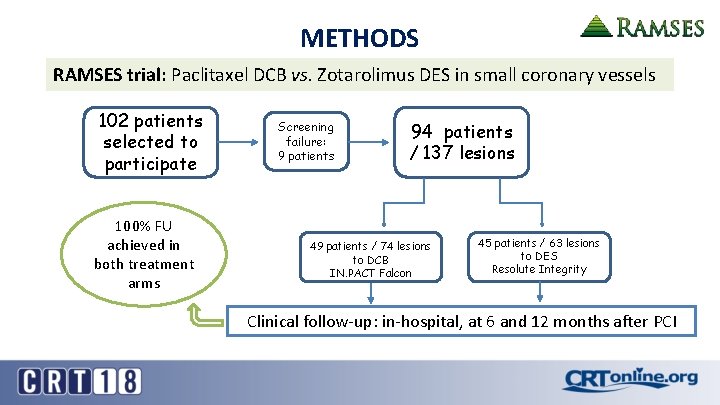
METHODS RAMSES trial: Paclitaxel DCB vs. Zotarolimus DES in small coronary vessels 102 patients selected to participate 100% FU achieved in both treatment arms Screening failure: 9 patients 94 patients / 137 lesions 49 patients / 74 lesions to DCB IN. PACT Falcon 45 patients / 63 lesions to DES Resolute Integrity Clinical follow-up: in-hospital, at 6 and 12 months after PCI

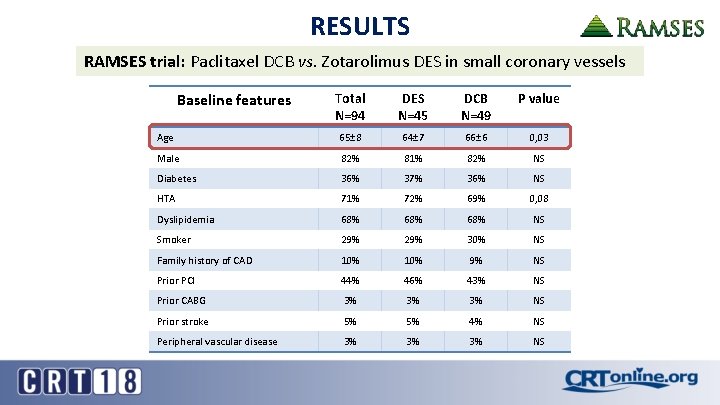
RESULTS RAMSES trial: Paclitaxel DCB vs. Zotarolimus DES in small coronary vessels Baseline features Total N=94 DES N=45 DCB N=49 P value Age 65± 8 64± 7 66± 6 0, 03 Male 82% 81% 82% NS Diabetes 36% 37% 36% NS HTA 71% 72% 69% 0, 08 Dyslipidemia 68% 68% NS Smoker 29% 30% NS Family history of CAD 10% 9% NS Prior PCI 44% 46% 43% NS Prior CABG 3% 3% 3% NS Prior stroke 5% 5% 4% NS Peripheral vascular disease 3% 3% 3% NS

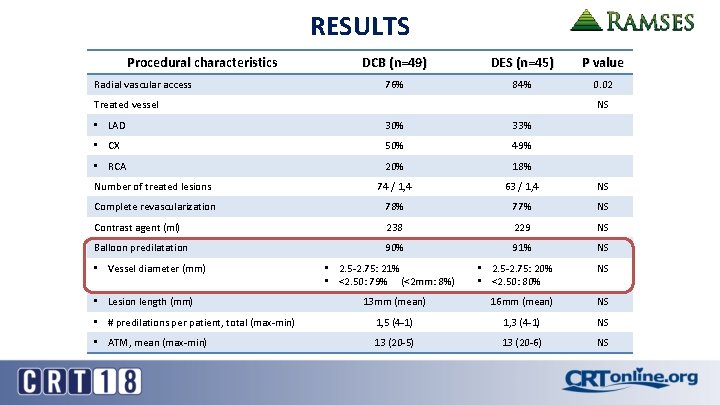
RESULTS Procedural characteristics Radial vascular access DCB (n=49) DES (n=45) P value 76% 84% 0. 02 Treated vessel NS • LAD 30% 33% • CX 50% 49% • RCA 20% 18% Number of treated lesions 74 / 1, 4 63 / 1, 4 NS Complete revascularization 78% 77% NS Contrast agent (ml) 238 229 NS Balloon predilatation 90% 91% NS • Vessel diameter (mm) • 2. 5 -2. 75: 20% • <2. 50: 80% NS 13 mm (mean) 16 mm (mean) NS • # predilations per patient, total (max-min) 1, 5 (4 -1) 1, 3 (4 -1) NS • ATM, mean (max-min) 13 (20 -5) 13 (20 -6) NS • Lesion length (mm) • 2. 5 -2. 75: 21% • <2. 50: 79% (<2 mm: 8%)

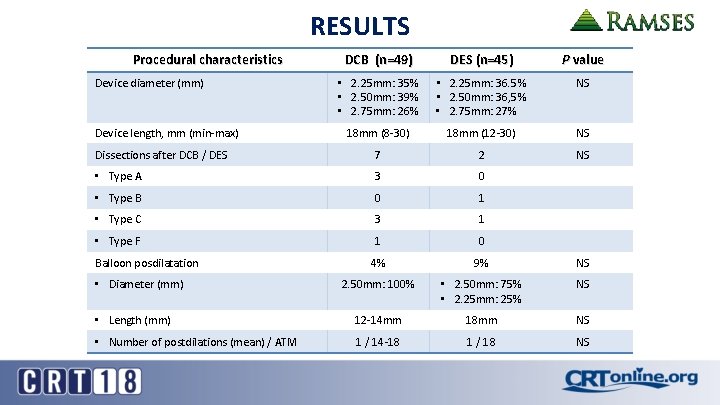
RESULTS Procedural characteristics DCB (n=49) DES (n=45) P value • 2. 25 mm: 35% • 2. 50 mm: 39% • 2. 75 mm: 26% • 2. 25 mm: 36. 5% • 2. 50 mm: 36, 5% • 2. 75 mm: 27% NS 18 mm (8 -30) 18 mm (12 -30) NS Dissections after DCB / DES 7 2 NS • Type A 3 0 • Type B 0 1 • Type C 3 1 • Type F 1 0 4% 9% NS 2. 50 mm: 100% • 2. 50 mm: 75% • 2. 25 mm: 25% NS • Length (mm) 12 -14 mm 18 mm NS • Number of postdilations (mean) / ATM 1 / 14 -18 1 / 18 NS Device diameter (mm) Device length, mm (min-max) Balloon posdilatation • Diameter (mm)

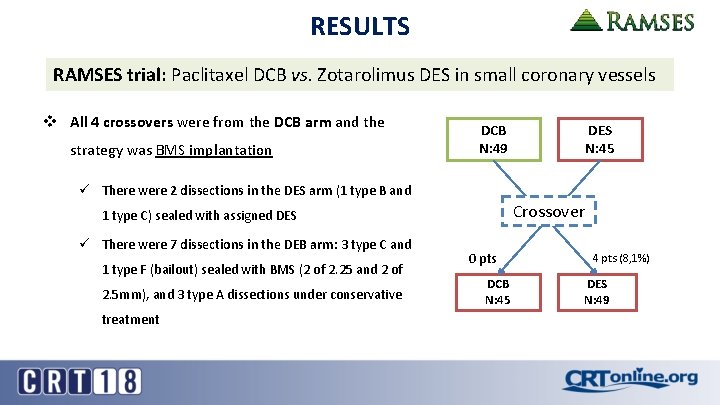
RESULTS RAMSES trial: Paclitaxel DCB vs. Zotarolimus DES in small coronary vessels v All 4 crossovers were from the DCB arm and the strategy was BMS implantation DCB N: 49 DES N: 45 ü There were 2 dissections in the DES arm (1 type B and Crossover 1 type C) sealed with assigned DES ü There were 7 dissections in the DEB arm: 3 type C and 1 type F (bailout) sealed with BMS (2 of 2. 25 and 2 of 2. 5 mm), and 3 type A dissections under conservative treatment 0 pts DCB N: 45 4 pts (8, 1%) DES N: 49

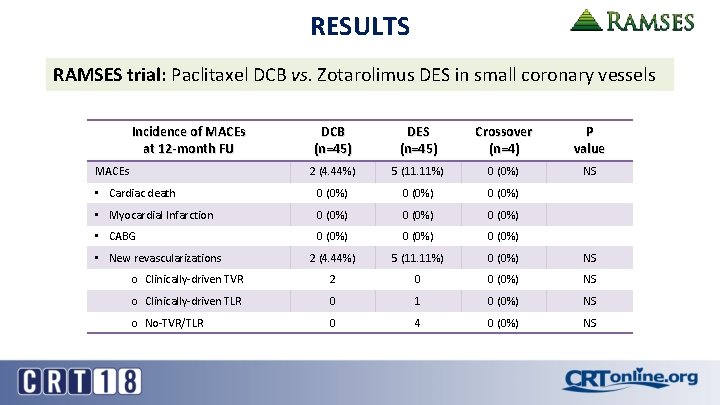
RESULTS RAMSES trial: Paclitaxel DCB vs. Zotarolimus DES in small coronary vessels Incidence of MACEs at 12 -month FU DCB (n=45) DES (n=45) Crossover (n=4) P value 2 (4. 44%) 5 (11. 11%) 0 (0%) NS • Cardiac death 0 (0%) • Myocardial Infarction 0 (0%) • CABG 0 (0%) 2 (4. 44%) 5 (11. 11%) 0 (0%) NS o Clinically-driven TVR 2 0 0 (0%) NS o Clinically-driven TLR 0 1 0 (0%) NS o No-TVR/TLR 0 4 0 (0%) NS MACEs • New revascularizations

CONCLUSIONS 1) The use of Paclitaxel-DCB for the treatment of de novo lesions in small coronary vessels seems to be as safe and effective as 2 nd gen. Zotarolimus-DES o Similar procedural success rates o No significant differences in periprocedural complications and MACEs at 12 -month FU o Low crossover rate in the DCB group 2) Incidence of MACEs is very low at 12 -months FU, in both groups 3) Larger population of patients and longer FU period is desirable