ENDEAVOR III Multicenter Randomized Trial 3 1 Randomization







- Slides: 14

ENDEAVOR III Multicenter Randomized Trial 3: 1 Randomization Single Blind – Single Vessel – No Staging Single De Novo Native Coronary Lesions Vessel Diameter: 2. 5 -3. 5 mm Lesion Length: 14 -27 mm Stent Lengths: 18 -33 mm (8/9) mm bailout Pre-dilatation required Endeavor Stent n = 327 Cypher Stent n = 109 N = 436 patients 30 U. S. sites Clinical Endpoints Clinical/MACE 30 d 6 mo 8 mo 9 mo 12 mo 2 yr 3 yr 4 yr Angio/IVUS QCA IVUS Primary Endpoint: In-segment late lumen loss by QCA at 8 months Secondary Endpoints: TLR, TVF at 9 months & ABR at 8 months Antiplatelet therapy for ³ 3 months 10 g Zotarolimus per mm stent length 5 yr

ENDEAVOR III Study Objectives (2003) • To determine whether results of the Endeavor DES in a United States PCI population were similar to those observed in Endeavor trials conducted internationally • To determine whether 8 month angiographic outcomes (in-segment late loss) with the Endeavor DES are non-inferior to the FDA-approved Cypher DES

ENDEAVOR III Outstanding Questions (2010) • Does higher late loss with Endeavor ZES stent (compared with Cypher SES) translate into greater late-term TLR beyond the period of angiographic follow-up? • What are the late-term safety outcomes of Endeavor ZES and Cypher SES? • Are Endeavor ZES outcomes consistent with late -term follow up observed in the overall Endeavor Clinical Trial program?

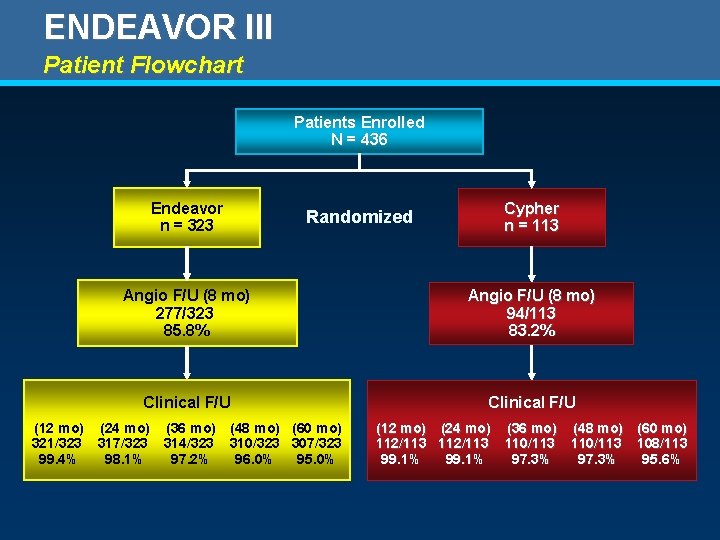
ENDEAVOR III Patient Flowchart Patients Enrolled N = 436 Endeavor n = 323 Randomized Cypher n = 113 Angio F/U (8 mo) 277/323 85. 8% Angio F/U (8 mo) 94/113 83. 2% Clinical F/U (12 mo) (24 mo) (36 mo) (48 mo) (60 mo) 321/323 317/323 314/323 310/323 307/323 99. 4% 98. 1% 97. 2% 96. 0% 95. 0% (12 mo) (24 mo) (36 mo) (48 mo) (60 mo) 112/113 110/113 108/113 99. 1% 97. 3% 95. 6%

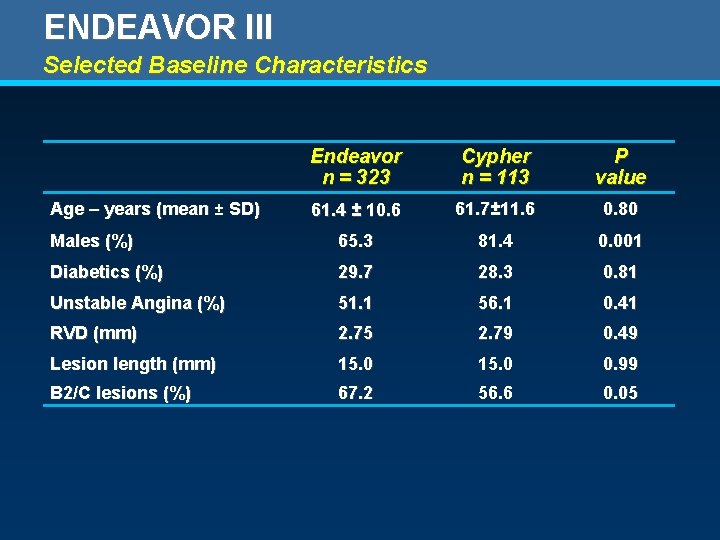
ENDEAVOR III Selected Baseline Characteristics Endeavor n = 323 Cypher n = 113 P value 61. 4 ± 10. 6 61. 7± 11. 6 0. 80 Males (%) 65. 3 81. 4 0. 001 Diabetics (%) 29. 7 28. 3 0. 81 Unstable Angina (%) 51. 1 56. 1 0. 41 RVD (mm) 2. 75 2. 79 0. 49 Lesion length (mm) 15. 0 0. 99 B 2/C lesions (%) 67. 2 56. 6 0. 05 Age – years (mean ± SD)

ENDEAVOR III Dual Antiplatelet Therapy (DAPT) Patients On DAPT At: 1 Year 2 Years 3 Years 4 Years 5 Years Endeavor 17. 5 (54/308) 5. 6 (17/304) 7. 5 (22/293) 6. 9 (20/289) 7. 0 (20/286) Cypher 16. 5 (18/109) 9. 3 (10/108) 9. 8 (10/102) 9. 3 (9/97) 6. 4 (6/94) Data presented as percentages.

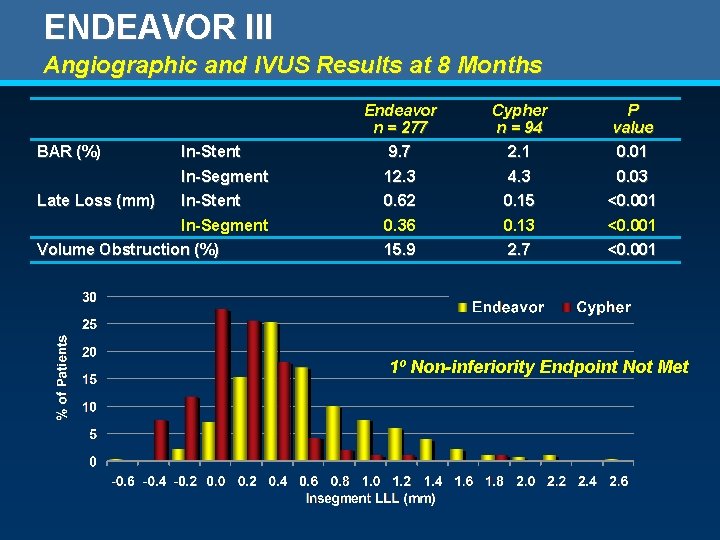
ENDEAVOR III Angiographic and IVUS Results at 8 Months BAR (%) Late Loss (mm) In-Stent In-Segment Volume Obstruction (%) Endeavor n = 277 9. 7 Cypher n = 94 2. 1 P value 0. 01 12. 3 0. 62 4. 3 0. 15 0. 03 <0. 001 0. 36 0. 13 <0. 001 15. 9 2. 7 <0. 001 1º Non-inferiority Endpoint Not Met

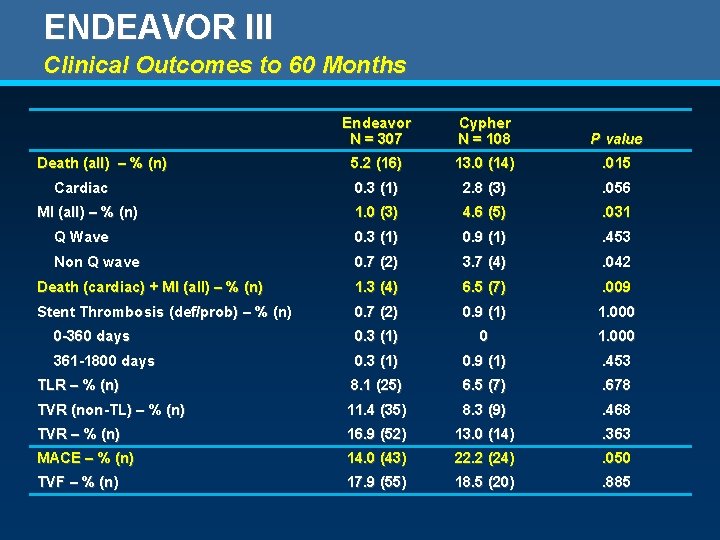
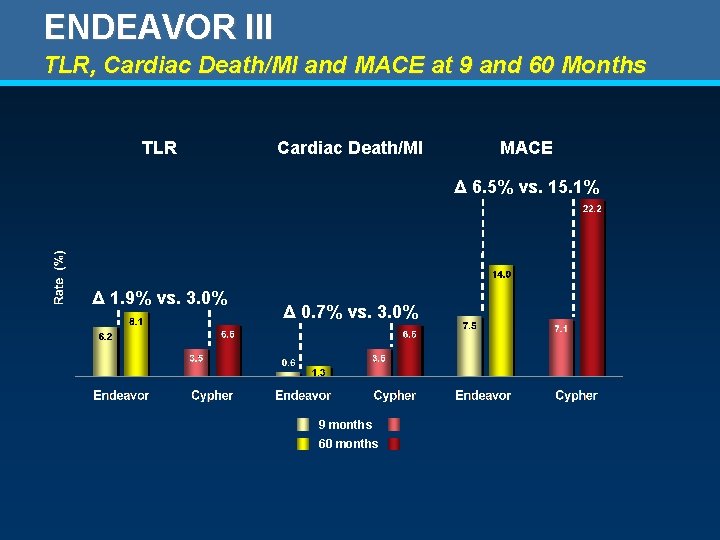
ENDEAVOR III Clinical Outcomes to 60 Months Endeavor N = 307 Cypher N = 108 P value 5. 2 (16) 13. 0 (14) . 015 0. 3 (1) 2. 8 (3) . 056 1. 0 (3) 4. 6 (5) . 031 Q Wave 0. 3 (1) 0. 9 (1) . 453 Non Q wave 0. 7 (2) 3. 7 (4) . 042 Death (cardiac) + MI (all) – % (n) 1. 3 (4) 6. 5 (7) . 009 Stent Thrombosis (def/prob) – % (n) 0. 7 (2) 0. 9 (1) 1. 000 0 -360 days 0. 3 (1) 0 1. 000 361 -1800 days 0. 3 (1) 0. 9 (1) . 453 TLR – % (n) 8. 1 (25) 6. 5 (7) . 678 TVR (non-TL) – % (n) 11. 4 (35) 8. 3 (9) . 468 TVR – % (n) 16. 9 (52) 13. 0 (14) . 363 MACE – % (n) 14. 0 (43) 22. 2 (24) . 050 TVF – % (n) 17. 9 (55) 18. 5 (20) . 885 Death (all) – % (n) Cardiac MI (all) – % (n)

ENDEAVOR III TLR, Cardiac Death/MI and MACE at 9 and 60 Months TLR Cardiac Death/MI MACE Δ 6. 5% vs. 15. 1% Δ 1. 9% vs. 3. 0% Δ 0. 7% vs. 3. 0% 9 months 60 months

ENDEAVOR III Cardiac Death/MI to 60 Months Cumulative Incidence of Cardiac Death or MI 15% £ 1 year > 1 year through 5 years Endeavor Cardiac Death/MI = 0. 62% Death/MI = 0. 67% Endeavor P = 0. 003 Cypher 10% 6. 5% 5% 1. 3% 0% CD/MI Endeavor Events n (CI%) Cypher # Events n (CI%) 0 360 720 1080 1440 Time after Initial Procedure (days) 1800 0 360 720 1080 1440 1800 323 321 314 306 298 290 2 (0. 6) 3 (1. 0) 4 (1. 3) 113 109 107 103 98 94 4 (3. 5) 6 (5. 5) 7 (6. 5)

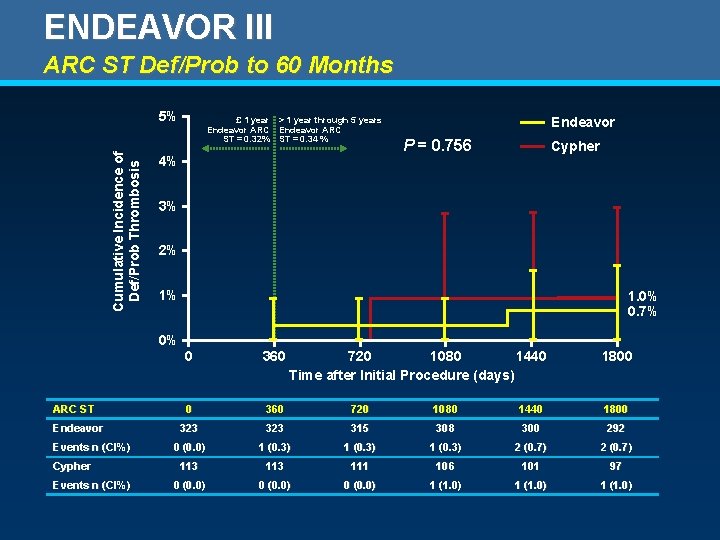
ENDEAVOR III ARC ST Def/Prob to 60 Months Cumulative Incidence of Def/Prob Thrombosis 5% £ 1 year > 1 year through 5 years Endeavor ARC ST = 0. 32% ST = 0. 34 % 4% Endeavor P = 0. 756 Cypher 3% 2% 1% 1. 0% 0. 7% 0% ARC ST Endeavor Events n (CI%) Cypher Events n (CI%) 0 360 720 1080 1440 Time after Initial Procedure (days) 1800 0 360 720 1080 1440 1800 323 315 308 300 292 0 (0. 0) 1 (0. 3) 2 (0. 7) 113 111 106 101 97 0 (0. 0) 1 (1. 0)

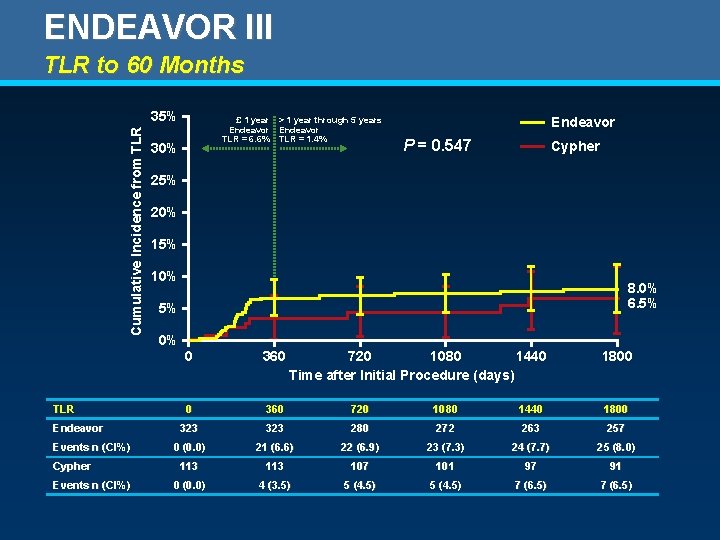
ENDEAVOR III TLR to 60 Months Cumulative Incidence from TLR 35% TLR Endeavor Events n (CI%) Cypher Events n (CI%) £ 1 year > 1 year through 5 years Endeavor TLR = 6. 6% TLR = 1. 4% 30% Endeavor P = 0. 547 Cypher 25% 20% 15% 10% 8. 0% 6. 5% 5% 0% 0 360 720 1080 1440 Time after Initial Procedure (days) 1800 0 360 720 1080 1440 1800 323 280 272 263 257 0 (0. 0) 21 (6. 6) 22 (6. 9) 23 (7. 3) 24 (7. 7) 25 (8. 0) 113 107 101 97 91 0 (0. 0) 4 (3. 5) 5 (4. 5) 7 (6. 5)

ENDEAVOR III Cumulative Incidence from MACE to 60 Months 35% £ 1 year Ø 1 year through 5 years Endeavor MACE = 7. 8% MACE = 5. 8% 30% Endeavor Events n (CI%) Cypher Events n (CI%) P = 0. 054 Cypher 25% 21. 8% 20% 15% 13. 6% 10% 5% 0% 360 MACE Endeavor 720 1080 1440 Time after Initial Procedure (days) 1800 0 360 720 1080 1440 1800 323 321 293 284 275 268 2 (0. 6) 25 (7. 8) 29 (9. 0) 35 (11. 0) 39 (12. 3) 43 (13. 6) 113 109 103 98 94 88 4 (3. 5) 9 (8. 0) 13 (11. 5) 16 (14. 3) 21 (18. 9) 24 (21. 8)

ENDEAVOR III Conclusion at 5 Years 1. In a trial designed to evaluate a primary angiographic endpoint, late lumen loss is greater with Endeavor ZES compared with the Cypher SES 2. Despite initially higher angiographic late loss with ZES, A. Rates of TLR beyond period of protocol-mandated angiographic surveillance remain stable B. Absolute differences between DES in clinical restenosis temporally decrease over late term 3. Recognizing limitations in sample size, late term (5 y) follow up of the Endeavor stent demonstrates A. Durability in efficacy (TLR) B. Very low rate of VLST (1 event) C. Compared with Cypher SES, emerging differences in cardiac death/MI and MACE achieving statistical significance 4. Late term EIII outcomes with the Endeavor ZES parallel the consistent safety and efficacy observed across the Endeavor Clinical Trials Program