Classification of Matter Two Classifications of Matter v















- Slides: 19

Classification of Matter

Two Classifications of Matter v. Pure Substance v. Mixture

Pure Substance • A pure substance has the same composition throughout and does not vary from sample to sample.

Classification of Pure Substances v. Elements v. Compounds

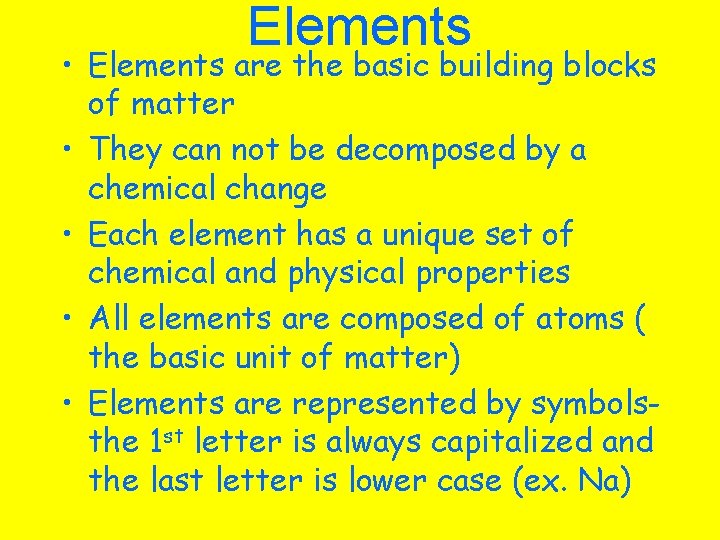
Elements • Elements are the basic building blocks of matter • They can not be decomposed by a chemical change • Each element has a unique set of chemical and physical properties • All elements are composed of atoms ( the basic unit of matter) • Elements are represented by symbolsthe 1 st letter is always capitalized and the last letter is lower case (ex. Na)

Examples: Sodium Chlorine Properties of Na: • Solid • Grey metal • Reacts violently with water Properties of Cl: • Gas • Yellow • Poisonous

Let’s explore more elements and their properties:

Compounds v. Compounds are composed of two or more elements. v. Elements in a compound have had their physical and chemical properties altered as a result of being chemically bonded via a chemical reaction. v. Compounds can be decomposed into two or more simpler compounds or elements by a chemical change.

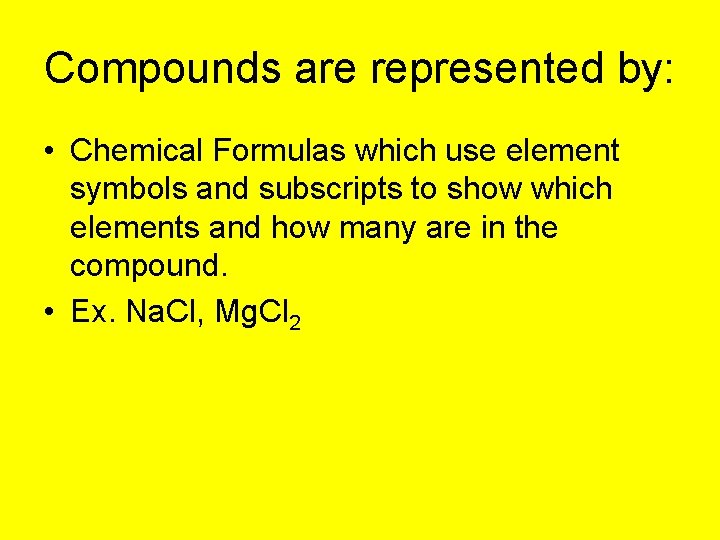
Compounds are represented by: • Chemical Formulas which use element symbols and subscripts to show which elements and how many are in the compound. • Ex. Na. Cl, Mg. Cl 2

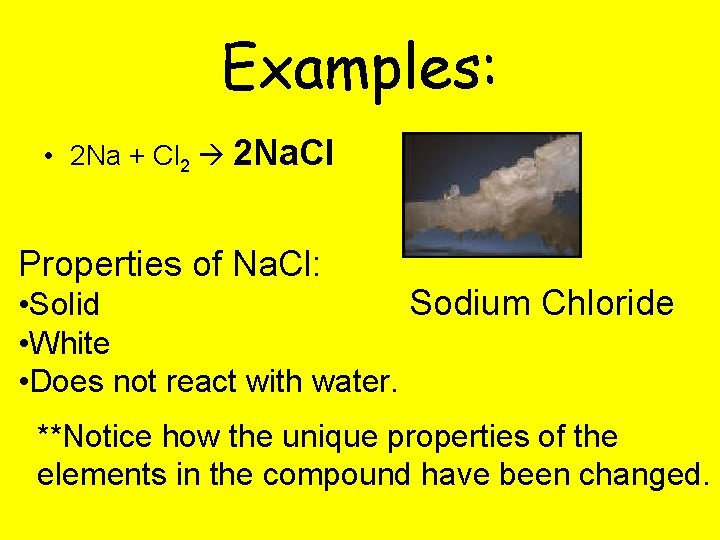
Examples: • 2 Na + Cl 2 2 Na. Cl Properties of Na. Cl: Sodium Chloride • Solid • White • Does not react with water. **Notice how the unique properties of the elements in the compound have been changed.

Mixtures • A mixture contains more than one substance. • The composition of a mixture can vary from sample to sample; however, each component of a mixture retains its own unique physical and chemical properties. • Mixtures can be separated by physical means.

Classification of Mixtures v. Homogeneous v. Heterogeneous

Homogeneous Mixtures • Homogeneous mixtures are also known as solutions. • They have a uniform composition and the components can not be seen as separate. Lemonade Vinegar

Heterogeneous Mixtures • Heterogeneous mixtures do not have a uniform composition and the components can be seen as separate. Granite Pizza

Separation Techniques • Filtration • Distillation • Crystallization • Chromatography

Filtrations • Used to separate heterogeneous mixtures. • The mixture is poured through a piece of paper which allows the liquid part to pass and catches the solid portion.

Distillations • Used to separate homogeneous mixtures. • This technique takes advantage of different boiling points.

Crystallization • Used to separate homogeneous mixtures. • The liquid portion is allowed to evaporate and the solid portion remains behind as a crystal.

Chromotography • Separation occurs by allowing a mixture to flow along a stationary substance (usually chromotography paper).
 Two classifications of matter
Two classifications of matter What are the two classifications of matter?
What are the two classifications of matter? Matter graphic organizer
Matter graphic organizer Scallops characteristics
Scallops characteristics The two aws classifications for fcaw electrodes are
The two aws classifications for fcaw electrodes are Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Strongly flavored vegetables
Strongly flavored vegetables 5 classification of vegetables
5 classification of vegetables Mildly flavored vegetables cooking methods
Mildly flavored vegetables cooking methods Subfamily rosoideae
Subfamily rosoideae Solanum nigrum classification
Solanum nigrum classification What is oblique triangle
What is oblique triangle Ct dph reportable events
Ct dph reportable events Carbohydrate classifications
Carbohydrate classifications Types of lightning
Types of lightning 818 dewey decimal
818 dewey decimal Draft horse organism classifications
Draft horse organism classifications Unsd classifications
Unsd classifications