Chemistry and Calculations for the Hydrolysis of Salts



- Slides: 8

Chemistry and Calculations for the Hydrolysis of Salts Paul Gilletti Ph. D. Mesa Community College Mesa, AZ 1

The ions of salts can have an influence on the p. H of a solution. Ions that come from a strong acid or base do not influence the p. H. WHY? Since strong acids and bases are 100% ionized in water, the ions are unable to reform the molecular acid or the base in water. HCl + H 2 O H 3 O+ + Cl- “one way arrow” Na. OH + H 2 O Na+ + OH- + H 2 O “one way arrow” Na. Cl is the salt that comes from a strong acid and a strong base. 2

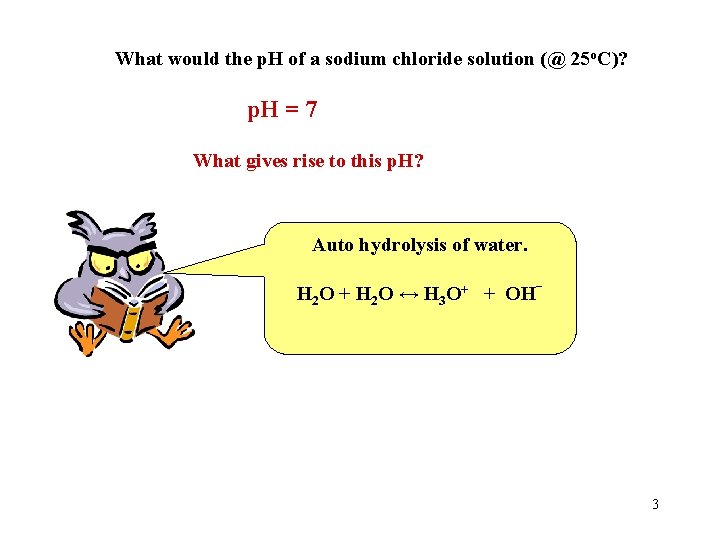
What would the p. H of a sodium chloride solution (@ 25 o. C)? p. H = 7 What gives rise to this p. H? Auto hydrolysis of water. H 2 O + H 2 O ↔ H 3 O+ + OH- 3

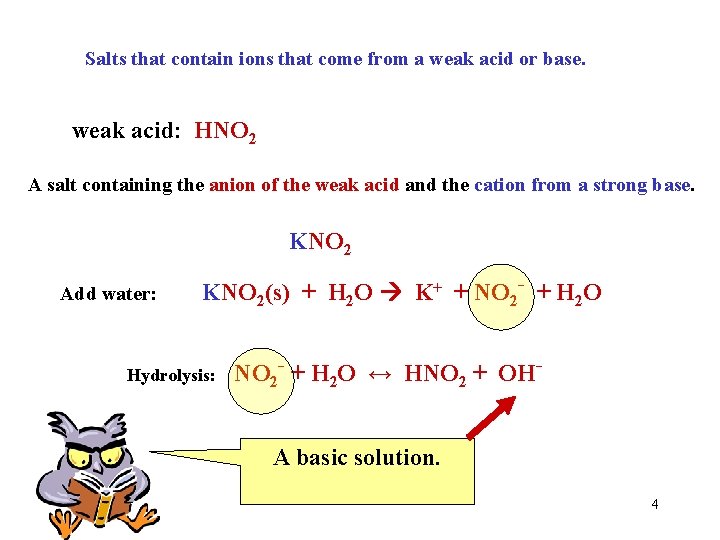
Salts that contain ions that come from a weak acid or base. weak acid: HNO 2 A salt containing the anion of the weak acid and the cation from a strong base. KNO 2 Add water: KNO 2(s) + H 2 O K+ + NO 2 - + H 2 O Hydrolysis: NO 2 - + H 2 O ↔ HNO 2 + OHA basic solution. 4

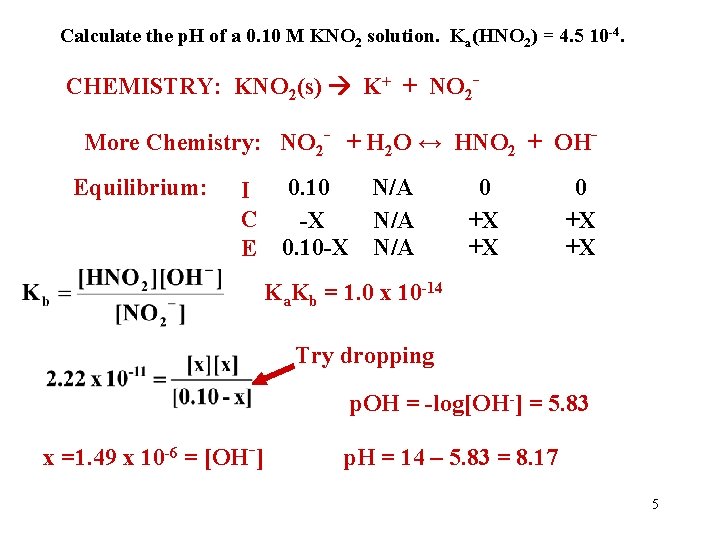
Calculate the p. H of a 0. 10 M KNO 2 solution. Ka(HNO 2) = 4. 5 10 -4. CHEMISTRY: KNO 2(s) K+ + NO 2 More Chemistry: NO 2 - + H 2 O ↔ HNO 2 + OHEquilibrium: 0. 10 N/A I C -X N/A E 0. 10 -X N/A 0 +X +X Ka. Kb = 1. 0 x 10 -14 Try dropping p. OH = -log[OH-] = 5. 83 x =1. 49 x 10 -6 = [OH-] p. H = 14 – 5. 83 = 8. 17 5

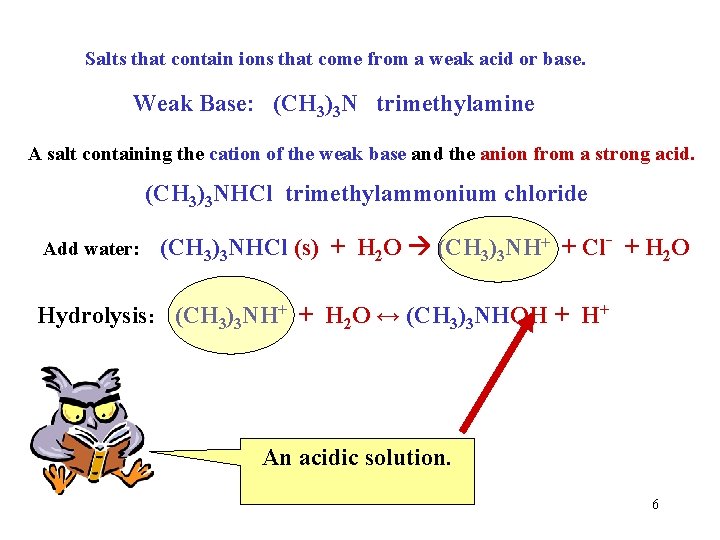
Salts that contain ions that come from a weak acid or base. Weak Base: (CH 3)3 N trimethylamine A salt containing the cation of the weak base and the anion from a strong acid. (CH 3)3 NHCl trimethylammonium chloride Add water: (CH 3)3 NHCl (s) + H 2 O (CH 3)3 NH+ + Cl- + H 2 O Hydrolysis: (CH 3)3 NH+ + H 2 O ↔ (CH 3)3 NHOH + H+ An acidic solution. 6

Calculate the p. H of a 0. 10 (CH 3)3 NHCl solution. Kb((CH 3)3 NHCl ) = 7. 4 10 -5. CHEMISTRY: (CH 3)3 NHCl(s) + H 2 O (CH 3)3 NH+ + Cl- + H 2 O More Chemistry: Equilibrium: (CH 3)3 NH+ + H 2 O ↔ (CH 3)3 NHOH- + H+ I 0. 10 C -x E 0. 10 -x N/A N/A 0 +x +x Ka. Kb = 1. 0 x 10 -14 Try dropping p. H = -log[H+] = 5. 43 x =3. 68 x 10 -6 = [H+] 7

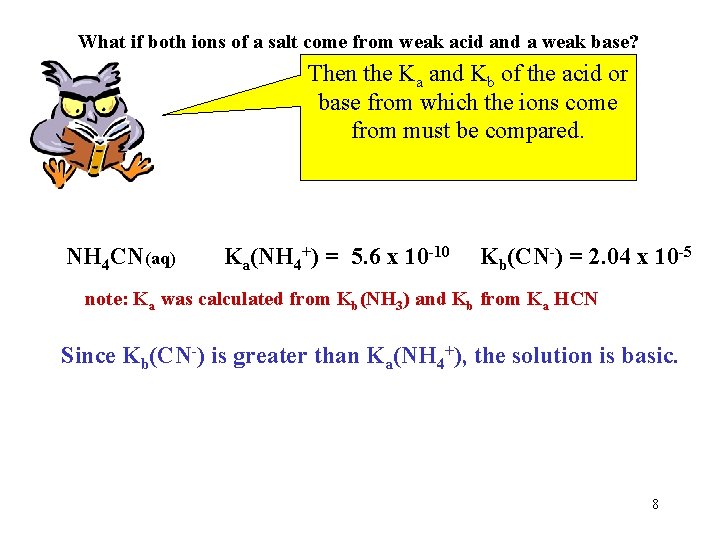
What if both ions of a salt come from weak acid and a weak base? Then the Ka and Kb of the acid or base from which the ions come from must be compared. NH 4 CN(aq) Ka(NH 4+) = 5. 6 x 10 -10 Kb(CN-) = 2. 04 x 10 -5 note: Ka was calculated from Kb(NH 3) and Kb from Ka HCN Since Kb(CN-) is greater than Ka(NH 4+), the solution is basic. 8