Chemical Physical Change Matter 1 What is matter













- Slides: 19

Chemical & Physical Change

Matter 1. What is matter? -anything that has mass and takes up space 2. What is mass? - the total amount of matter in an object; the mass of an object equals the total mass of its parts

3. What are some tools used to measure mass and see mass? A. balance scale- measures the amount of mass B. magnifying glass- helps smaller objects appear larger C. microscope- helps smaller objects appear larger

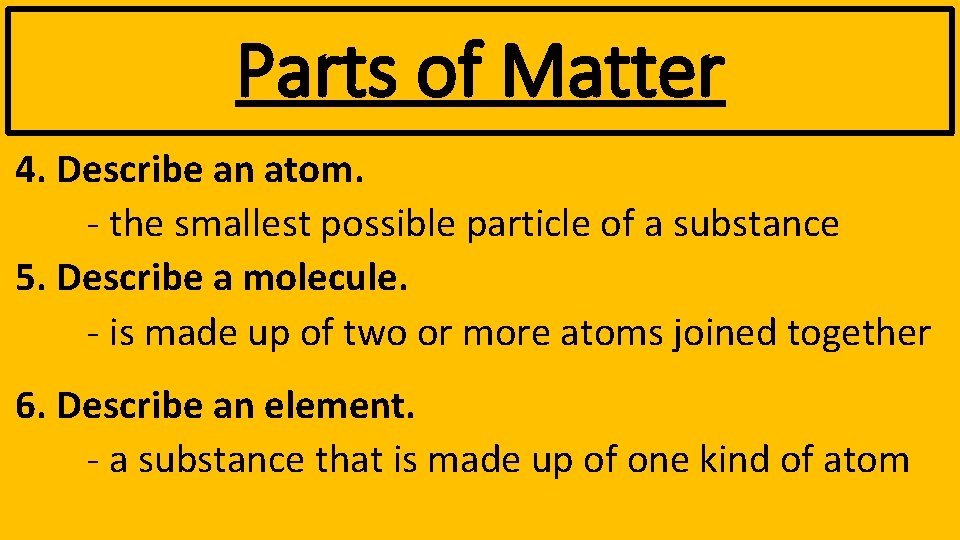
Parts of Matter 4. Describe an atom. - the smallest possible particle of a substance 5. Describe a molecule. - is made up of two or more atoms joined together 6. Describe an element. - a substance that is made up of one kind of atom

7. Show some different examples of elements.

7. (Continued) List of elements (paste-in) 8. What are the two types of metals? A. metals- shiny, can be drawn into wires; 75% of all elements are metals; Examples: iron, gold, and silver B. nonmetals - dull, brittle, some are usually a gas Examples: carbon,

9. What does it mean to malleable and ductile? A. malleable are metals that are easy to shape or form B. ductile are metals that can be pulled into thin strands, such as wires without breaking

States of Matter 10. What are three states of matter? A. Liquid- particles of water are close to each other and they move quickly and slide past each other quickly B. Solid- particles of ice are locked in place, although still vibrating C. Gas – particles are far apart and are moving quickly

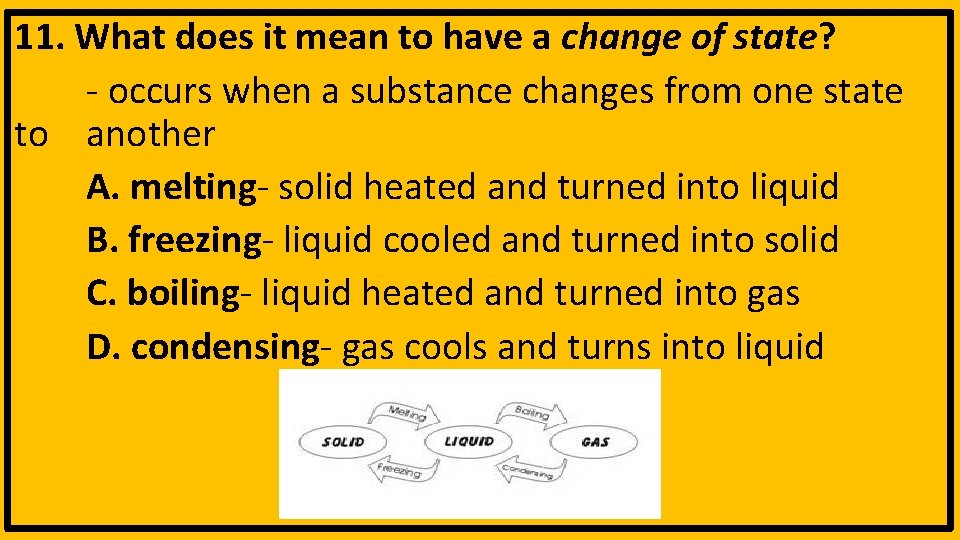
11. What does it mean to have a change of state? - occurs when a substance changes from one state to another A. melting- solid heated and turned into liquid B. freezing- liquid cooled and turned into solid C. boiling- liquid heated and turned into gas D. condensing- gas cools and turns into liquid

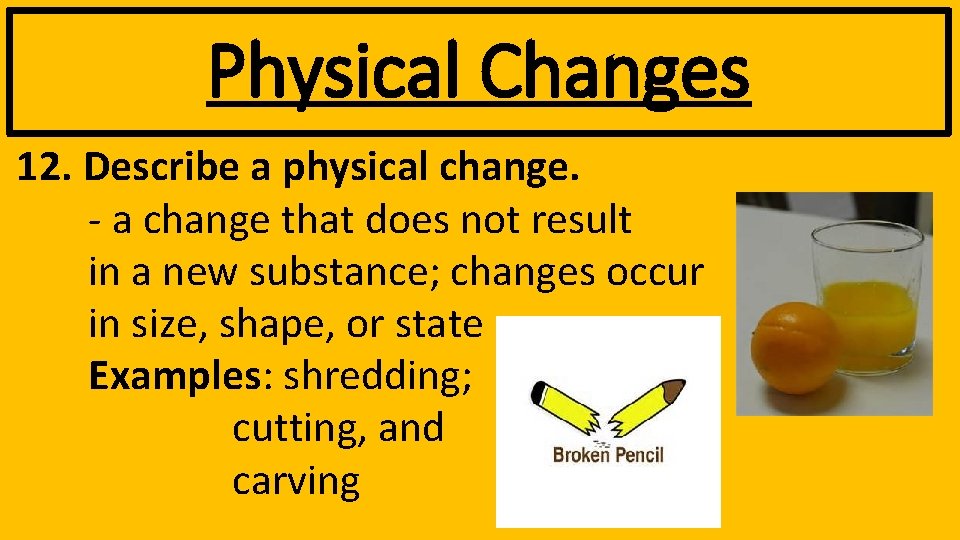
Physical Changes 12. Describe a physical change. - a change that does not result in a new substance; changes occur in size, shape, or state Examples: shredding; cutting, and carving

13. Explain the term physical property. - a feature of matter that you can recognize with your senses 14. Explain the term texture. - the feel of a surface.

Mixtures 15. Describe a mixture. - is two or more substances that are combined without being changed; the substances in a mixture aren’t permanently combined, and their properties don’t change.

16. What is an example of a mixture of solids? - salad, soil, assorted candy, Chex Mix 17. What is an example of a mixture of liquids? - lemonade, chocolate milk, soda, Kool aid 18. What is an example of a mixture of gas? - the air we breath

19. How can you separate mixtures? A. filters- a paper, plastic, metal or other type of screen with small holes in it. B. magnets- separates metals; removes iron that are attracted to magnet C. boiling – separates sugar, salt from water D. floating- salt and pepper make a solid mixture; put it in a glass the salt falls and the pepper floats

Chemical Changes 20. Explain the difference between physical properties and chemical properties. A. Physical properties are traits that involve a substance by itself. B. Chemical properties are properties that involve how a substance interacts with other substances

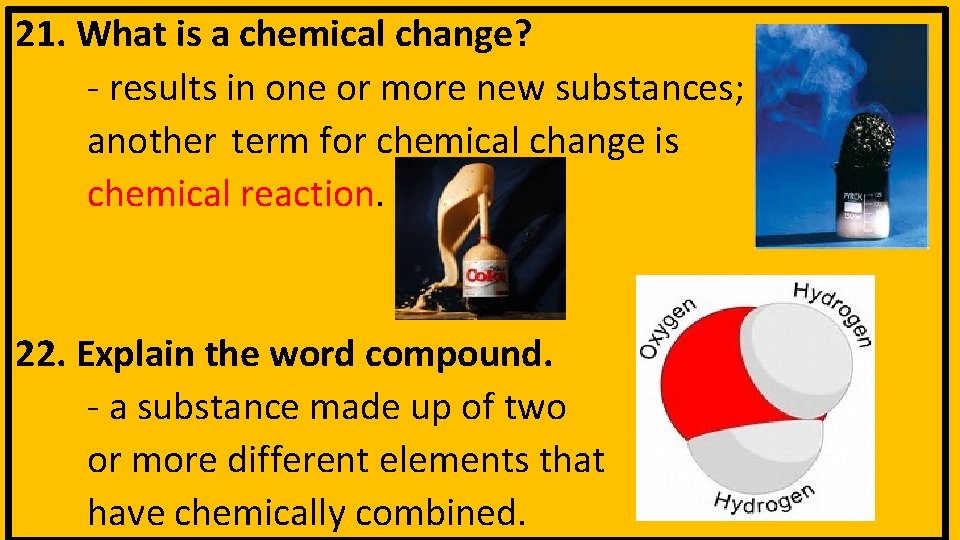
21. What is a chemical change? - results in one or more new substances; another term for chemical change is chemical reaction. 22. Explain the word compound. - a substance made up of two or more different elements that have chemically combined.

23. What do chemical reactions have to do with compounds? - compounds form when elements combine to form new substances during chemical reactions

24. How are chemical changes recognized? Clues to Chemical Change Clue Example Description Color Change Bread dough baking Changes from white to brown Smell Eggs rotting Gives off a terrible smell New Physical Property Iron rusting Substance Given Off Wood burning Changes from hard and silvery to brittle and reddish brown Smoke is released into the air Heat Given off Sulfur burning Fire is hot

25. Explain the term law of conservation of mass. - this law states that matter can never be created or destroyed, atoms may rearrange in reactions, but they never disappear
 What is a physical change
What is a physical change Chemical change examples pictures
Chemical change examples pictures Whats the difference between physical and chemical change
Whats the difference between physical and chemical change Physical and chemical changes examples
Physical and chemical changes examples Spare change physical versus chemical change
Spare change physical versus chemical change Whats the difference between a physical and chemical change
Whats the difference between a physical and chemical change How does a physical change differ from a chemical change? *
How does a physical change differ from a chemical change? * An example of a physical change
An example of a physical change Chopping wood is a physical or chemical change
Chopping wood is a physical or chemical change Eating food physical or chemical change
Eating food physical or chemical change Undesirable change in the physical chemical
Undesirable change in the physical chemical Is baking cookies a chemical change
Is baking cookies a chemical change Aluminum foil is cut in half chemical or physical change
Aluminum foil is cut in half chemical or physical change Pouring milk on your oatmeal physical or chemical
Pouring milk on your oatmeal physical or chemical Physical changes in female during puberty
Physical changes in female during puberty Is blue color physical or chemical property
Is blue color physical or chemical property Charcoal in a fire turns to ash after several hours
Charcoal in a fire turns to ash after several hours Physcial change
Physcial change Dyeing fabric physical or chemical change
Dyeing fabric physical or chemical change Is garbage rotting a physical or chemical change
Is garbage rotting a physical or chemical change