CHAPTER 5 Reactions in Aqueous Solution Homework for



![p. H l p. H = -log [H+] l p. OH = - log p. H l p. H = -log [H+] l p. OH = - log](https://slidetodoc.com/presentation_image/3dc40ba7c8afd9a9677f1bf5f5518028/image-6.jpg)


- Slides: 11

CHAPTER 5 Reactions in Aqueous Solution Homework for Chapter 5 problems posted due Feb 26 Know: Table 5. 2, pg 182 Use: Figure 5. 3, page 179 Chem 105 Chpt 4 Lsn 12 1

Road Map l Where we were l Classifying reactions l Oxidation reactions l Where we are going l Oxidation reactions l Measuring concentrations of compounds in solution l p. H l Stoichiometry of reactions in aqueous solution 2 Chem 105 Chpt 4 Lsn 12

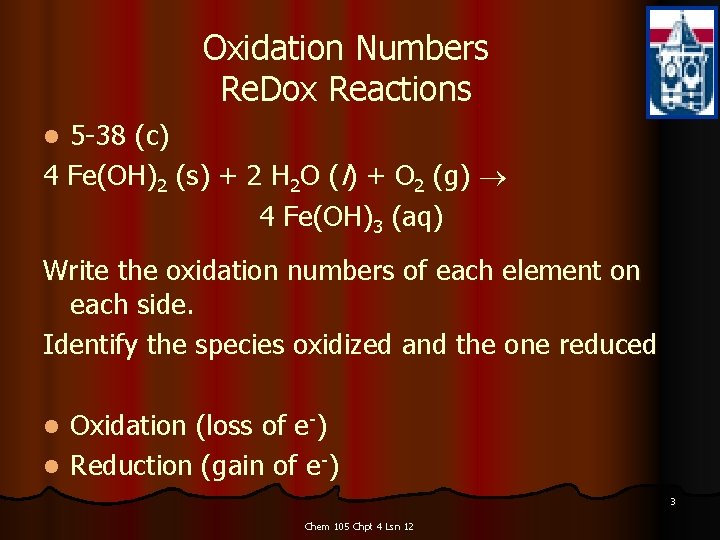
Oxidation Numbers Re. Dox Reactions 5 -38 (c) 4 Fe(OH)2 (s) + 2 H 2 O (l) + O 2 (g) 4 Fe(OH)3 (aq) l Write the oxidation numbers of each element on each side. Identify the species oxidized and the one reduced Oxidation (loss of e-) l Reduction (gain of e-) l 3 Chem 105 Chpt 4 Lsn 12

5. 8 Measuring Concentrations l How many moles of H+(aq) are present in 451 m. L of 3. 20 M hydrobromic acid? l 1. 44 mol H+ l 5 -46 What volume 2. 06 M KMn. O 4, in liters, contains 322 g of solute? l 0. 989 L 4 Chem 105 Chpt 4 Lsn 12

Preparing solutions l 5 -50 What mass of oxalic acid, H 2 C 2 O 4, is required to prepare 250. m. L of a solution that has a concentration or 0. 15 M H 2 C 2 O 4 ? l 3. 4 g H 2 C 2 O 4 5 Chem 105 Chpt 4 Lsn 12
![p H l p H log H l p OH log p. H l p. H = -log [H+] l p. OH = - log](https://slidetodoc.com/presentation_image/3dc40ba7c8afd9a9677f1bf5f5518028/image-6.jpg)
p. H l p. H = -log [H+] l p. OH = - log [OH-] l Kw = [H+][OH-] = 1. 00 x 10 -14 l p. H + p. OH = 14 l 5 -56 A saturated solution of milk of magnesia, Mg(OH)2, has a p. H of 10. 5. What is the hydrogen ion concentration of the solution? Is the solution acidic or basic? l 3 x 10 -11 l basic Chem 105 Chpt 4 Lsn 12 6

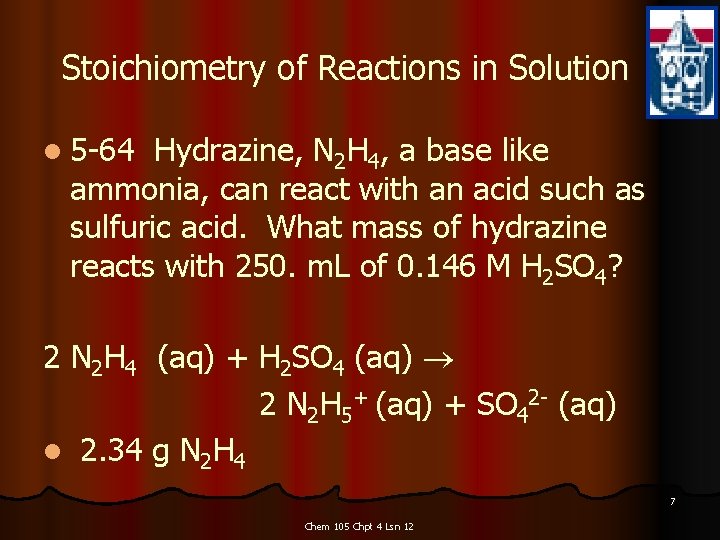
Stoichiometry of Reactions in Solution l 5 -64 Hydrazine, N 2 H 4, a base like ammonia, can react with an acid such as sulfuric acid. What mass of hydrazine reacts with 250. m. L of 0. 146 M H 2 SO 4? 2 N 2 H 4 (aq) + H 2 SO 4 (aq) 2 N 2 H 5+ (aq) + SO 42 - (aq) l 2. 34 g N 2 H 4 7 Chem 105 Chpt 4 Lsn 12

Challenge l 5 - 72 l 5 - 75 l 5 -76 l First 3 teams (max 4 members) to correctly solve the problem receive 5 bonus points 8 Chem 105 Chpt 4 Lsn 12

Chapter 6 Principles of Reactivity: Energy and Chemical Reactivity l Energy transfer Calorie burning, gravitational, chemical, electrostatic l Heat- mostly seen in chemical processes l l l Thermodynamics – transfer of heat between objects; science of heat and work Energy: Some basic principles – capacity to do work Kinetic – energy of motion l Potential – energy of position l l Conservation of energy (aka First law of thermodynamics) l Total energy of universe is constant Chem 105 Chpt 4 Lsn 12 9

Energy: Some basic principles – capacity to do work l Systems and surroundings l Thermal equilibrium – system and surroundings reach same temperature l Exothermic (heat out of system) l Endothermic (heat into system) 10 Chem 105 Chpt 4 Lsn 12

Next Lesson l Chapter 6 11 Chem 105 Chpt 4 Lsn 12