Ch 4 Electrons in Atoms I Waves Particles




- Slides: 13

Ch. 4 - Electrons in Atoms I. Waves & Particles (p. 91 - 94)

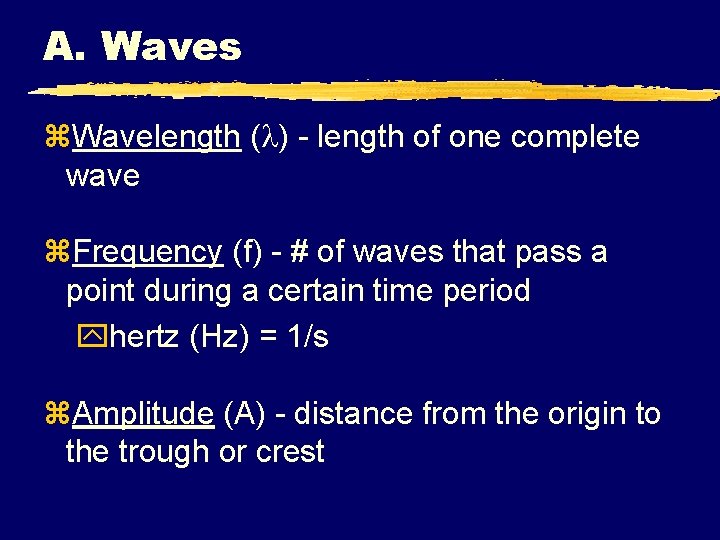
A. Waves z. Wavelength ( ) - length of one complete wave z. Frequency (f) - # of waves that pass a point during a certain time period yhertz (Hz) = 1/s z. Amplitude (A) - distance from the origin to the trough or crest

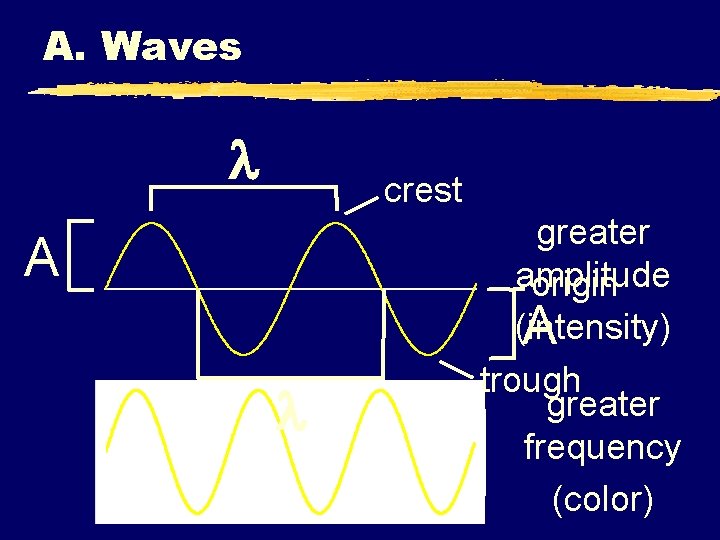
A. Waves crest A greater amplitude origin (intensity) A trough greater frequency (color)

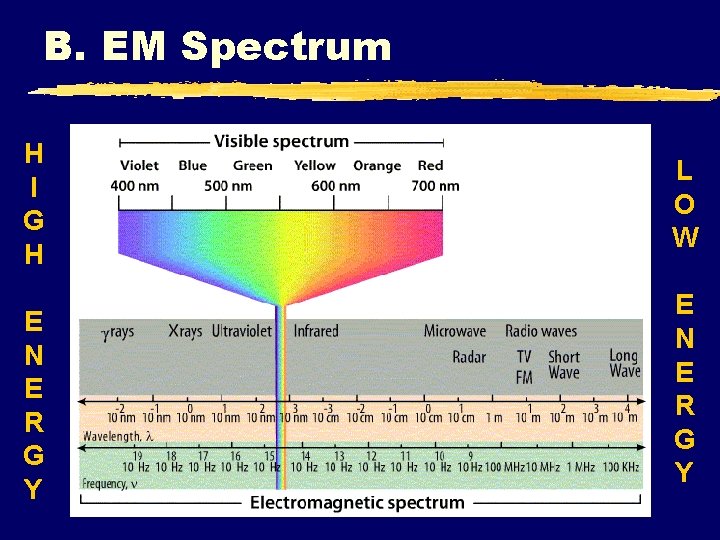
B. EM Spectrum H I G H E N E R G Y L O W E N E R G Y

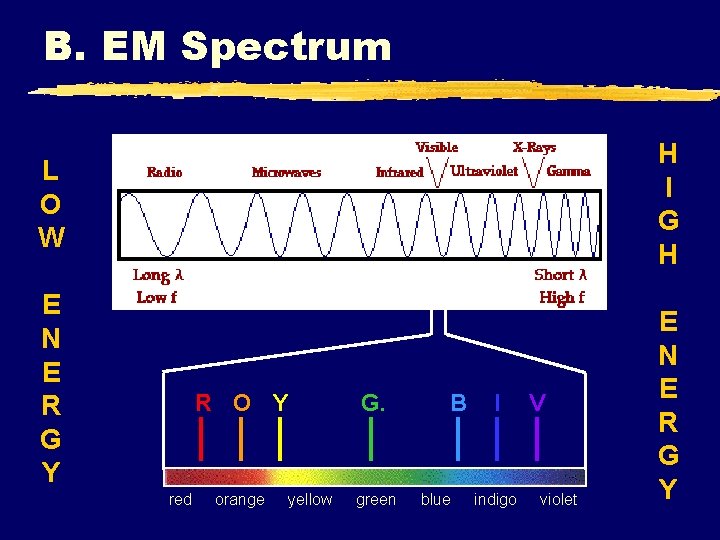
B. EM Spectrum H I G H L O W E N E R G Y red R O Y G. orange green yellow B blue I indigo V violet E N E R G Y

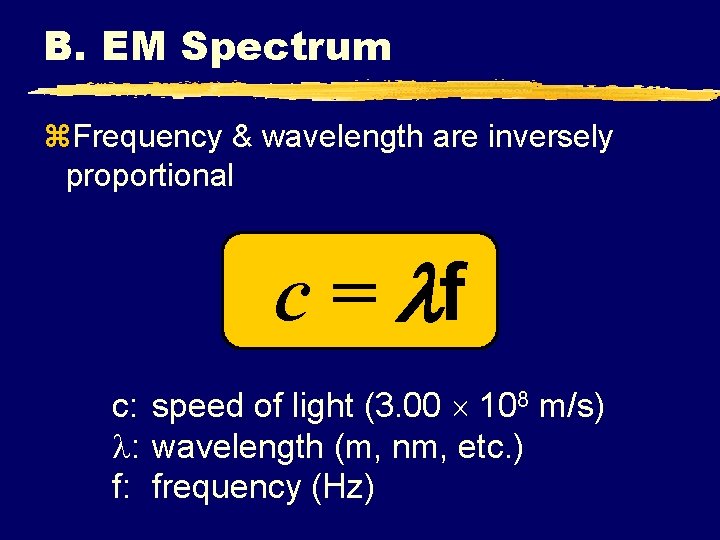
B. EM Spectrum z. Frequency & wavelength are inversely proportional c = f c: speed of light (3. 00 108 m/s) : wavelength (m, nm, etc. ) f: frequency (Hz)

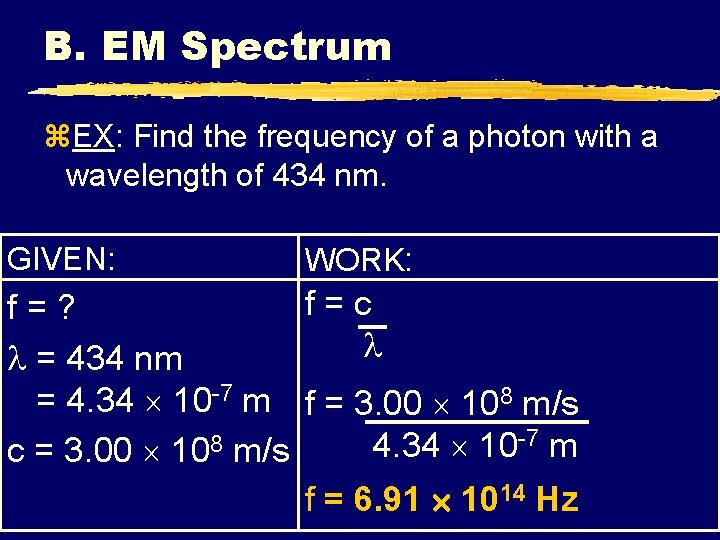
B. EM Spectrum z. EX: Find the frequency of a photon with a wavelength of 434 nm. GIVEN: WORK: f=c f=? = 434 nm = 4. 34 10 -7 m f = 3. 00 108 m/s -7 m 8 4. 34 10 c = 3. 00 10 m/s f = 6. 91 1014 Hz

C. Quantum Theory z. Planck (1900) y. Observed - emission of light from hot objects y. Concluded - energy is emitted in small, specific amounts (quanta) y. Quantum - minimum amount of energy change

C. Quantum Theory z. Planck (1900) vs. Classical Theory Quantum Theory

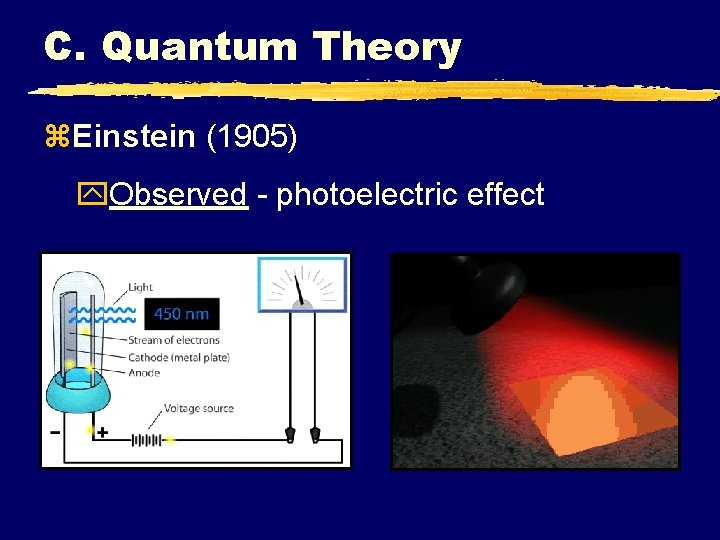
C. Quantum Theory z. Einstein (1905) y. Observed - photoelectric effect

C. Quantum Theory z. Einstein (1905) y. Concluded - light has properties of both waves and particles “wave-particle duality” y. Photon - particle of light that carries a quantum of energy

C. Quantum Theory z. The energy of a photon is proportional to its frequency. E = hf E: energy (J, joules) h: Planck’s constant (6. 6262 10 -34 J·s) f: frequency (Hz)

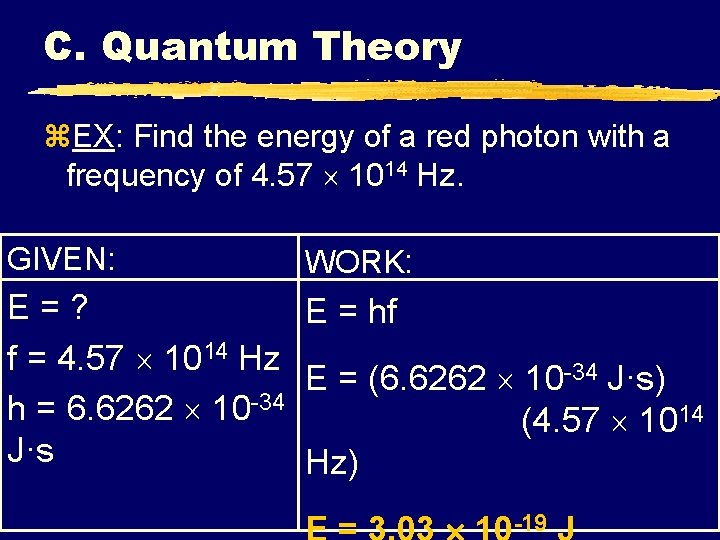
C. Quantum Theory z. EX: Find the energy of a red photon with a frequency of 4. 57 1014 Hz. GIVEN: WORK: E=? E = hf f = 4. 57 1014 Hz E = (6. 6262 10 -34 J·s) h = 6. 6262 10 -34 (4. 57 1014 J·s Hz) -19