CH 18 Charged Particles and Electric Forces of



- Slides: 26

CH 18: Charged Particles and Electric Forces of Charged Particles on Other Charged Particles

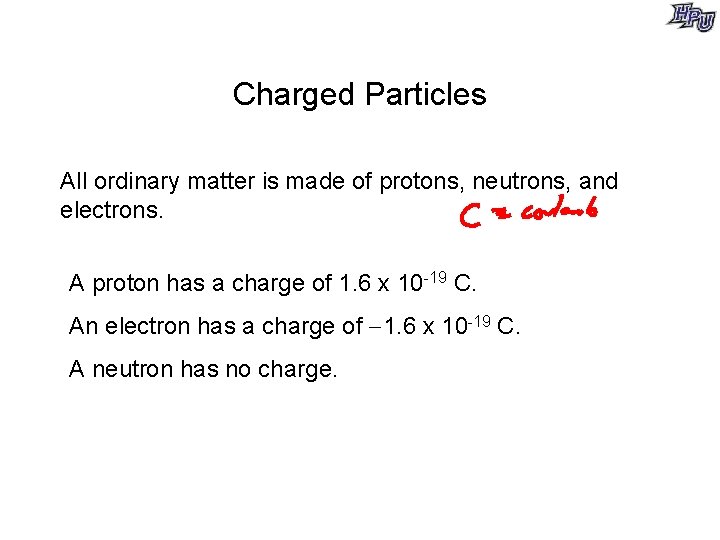
Charged Particles All ordinary matter is made of protons, neutrons, and electrons. A proton has a charge of 1. 6 x 10 -19 C. An electron has a charge of 1. 6 x 10 -19 C. A neutron has no charge.

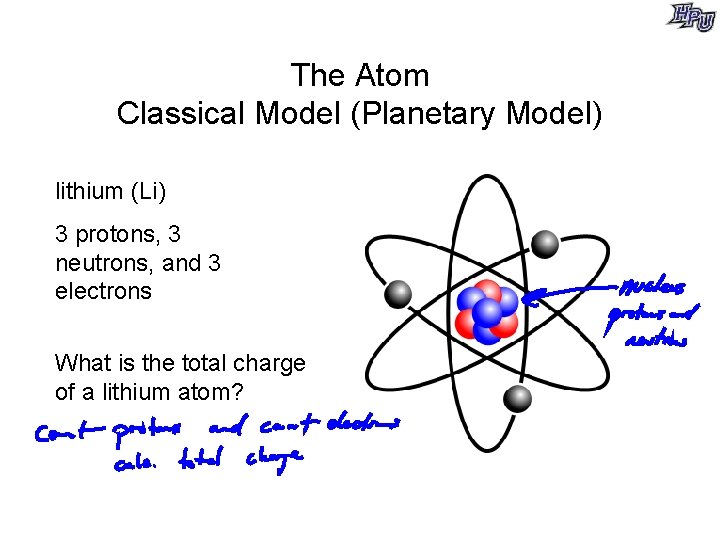
The Atom Classical Model (Planetary Model) lithium (Li) 3 protons, 3 neutrons, and 3 electrons What is the total charge of a lithium atom?

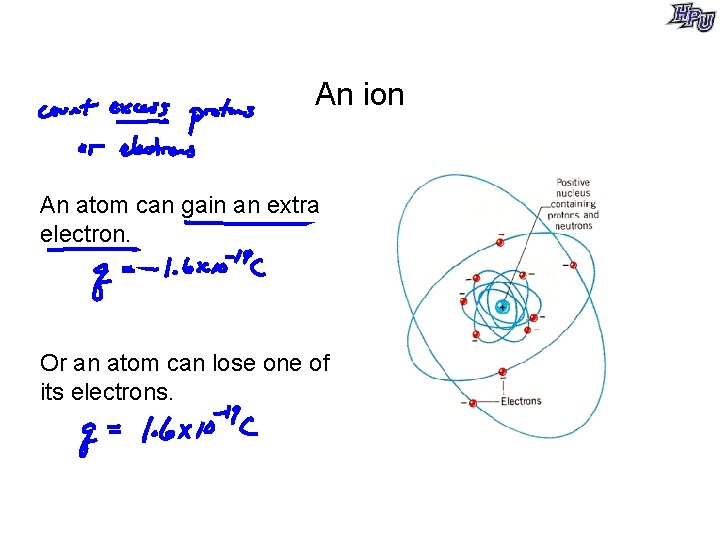
An ion An atom can gain an extra electron. Or an atom can lose one of its electrons.

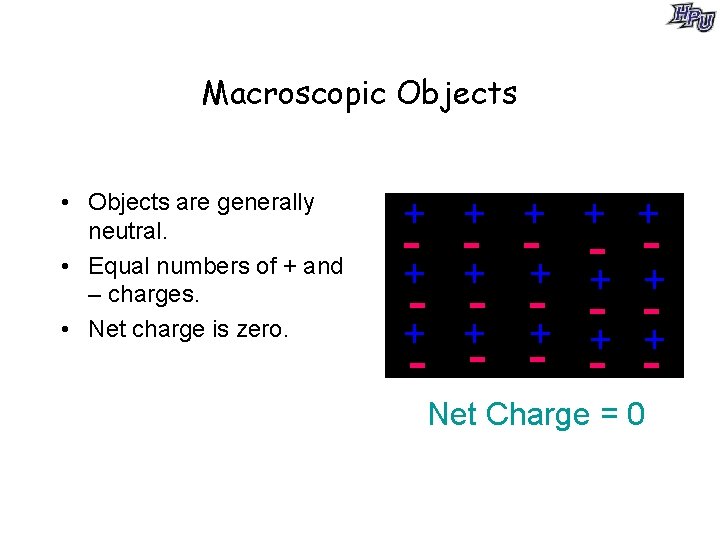
Macroscopic Objects • Objects are generally neutral. • Equal numbers of + and – charges. • Net charge is zero. + + + + + - - - - + + + - - Net Charge = 0

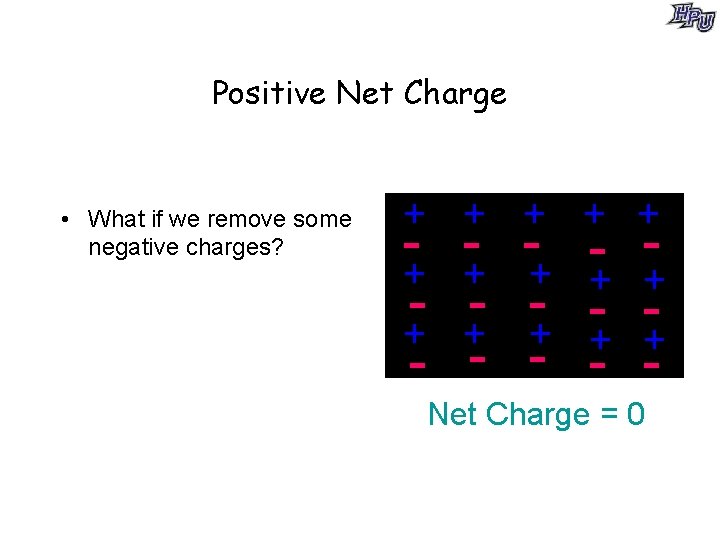
Positive Net Charge • What if we remove some negative charges? + + + + + - - - - + + + - - Net Charge = 0

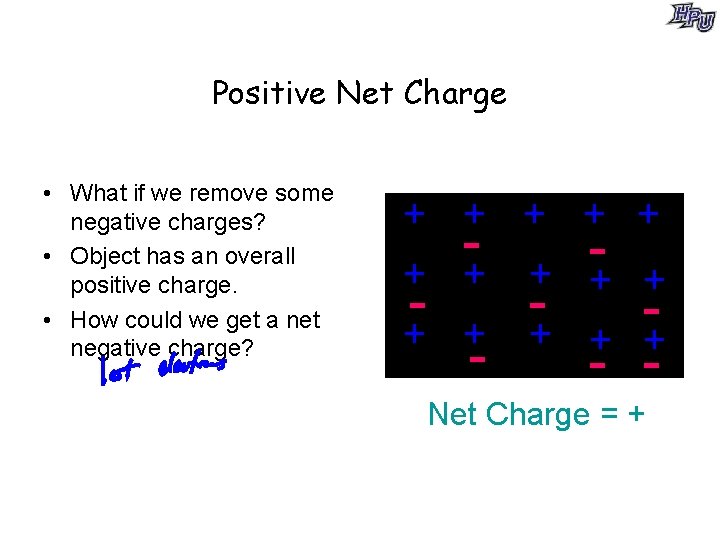
Positive Net Charge • What if we remove some negative charges? • Object has an overall positive charge. • How could we get a net negative charge? + + + + - - - Net Charge = +

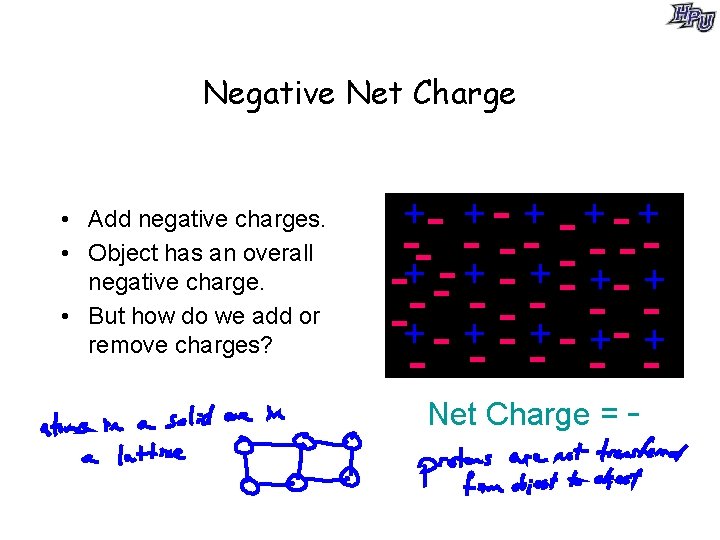
Negative Net Charge • Add negative charges. • Object has an overall negative charge. • But how do we add or remove charges? +- + - + -- - - - -+ -- + - +- + -+ - + - -+ - - Net Charge = -

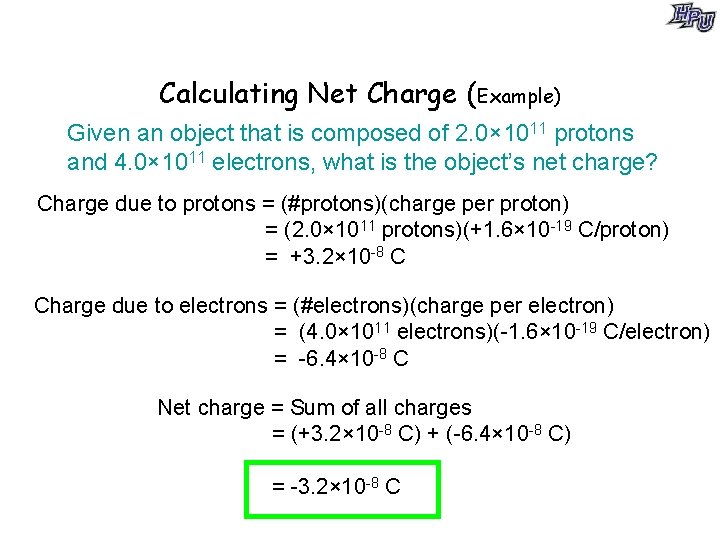
Calculating Net Charge (Example) Given an object that is composed of 2. 0× 1011 protons and 4. 0× 1011 electrons, what is the object’s net charge? Charge due to protons = (#protons)(charge per proton) = (2. 0× 1011 protons)(+1. 6× 10 -19 C/proton) = +3. 2× 10 -8 C Charge due to electrons = (#electrons)(charge per electron) = (4. 0× 1011 electrons)(-1. 6× 10 -19 C/electron) = -6. 4× 10 -8 C Net charge = Sum of all charges = (+3. 2× 10 -8 C) + (-6. 4× 10 -8 C) = -3. 2× 10 -8 C

Poll If you rub a balloon on your head, some electrons in the atoms of your hair move to the atoms of the latex balloon. Is the charge of the balloon, positive, negative, or zero? 1. positive 2. negative 3. zero

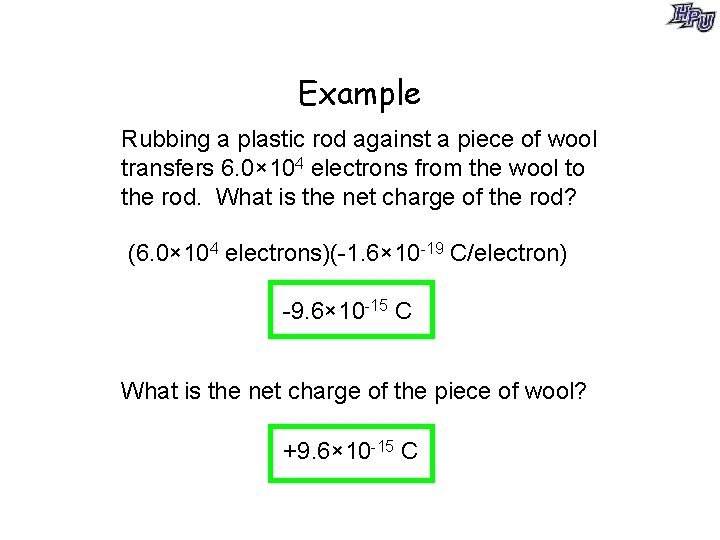
Example Rubbing a plastic rod against a piece of wool transfers 6. 0× 104 electrons from the wool to the rod. What is the net charge of the rod? (6. 0× 104 electrons)(-1. 6× 10 -19 C/electron) -9. 6× 10 -15 C What is the net charge of the piece of wool? +9. 6× 10 -15 C

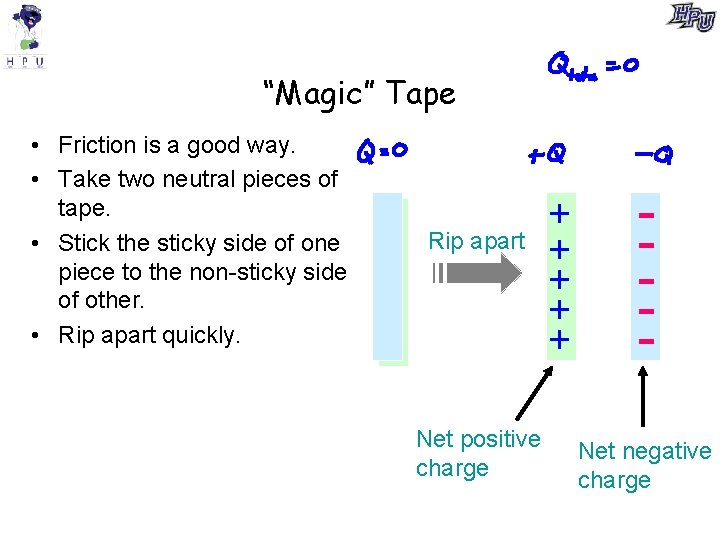
“Magic” Tape • Friction is a good way. • Take two neutral pieces of tape. • Stick the sticky side of one piece to the non-sticky side of other. • Rip apart quickly. Rip apart Net positive charge + + + Net negative charge

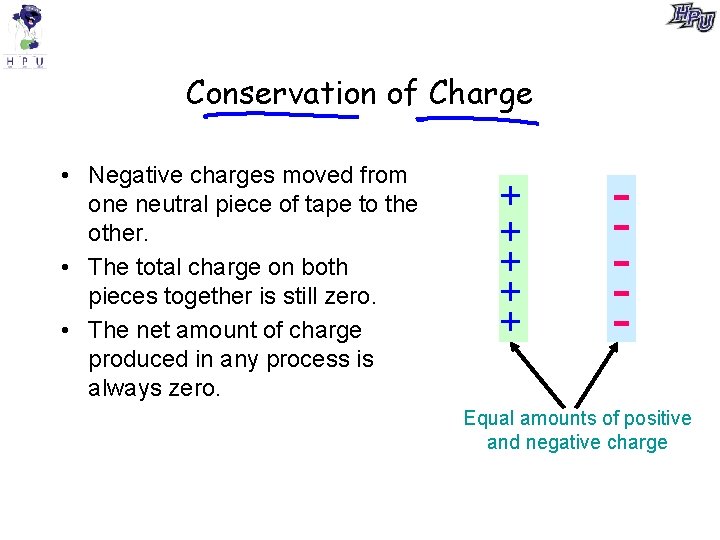
Conservation of Charge • Negative charges moved from one neutral piece of tape to the other. • The total charge on both pieces together is still zero. • The net amount of charge produced in any process is always zero. + + + - Equal amounts of positive and negative charge

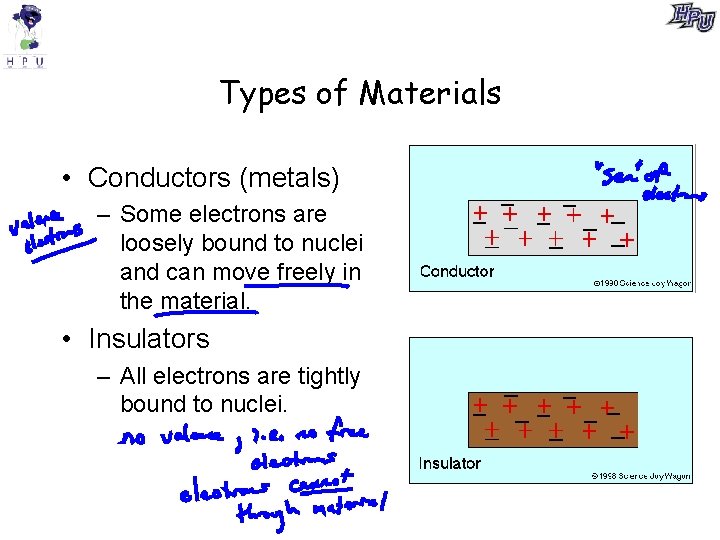
Types of Materials • Conductors (metals) – Some electrons are loosely bound to nuclei and can move freely in the material. • Insulators – All electrons are tightly bound to nuclei.

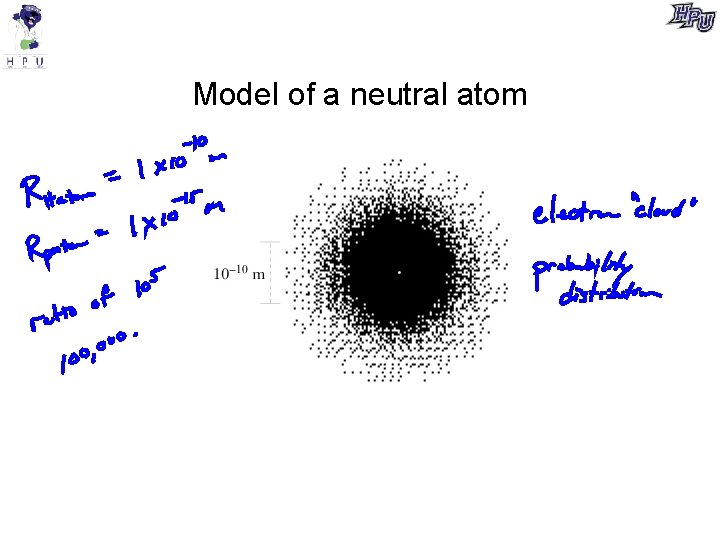
Model of a neutral atom

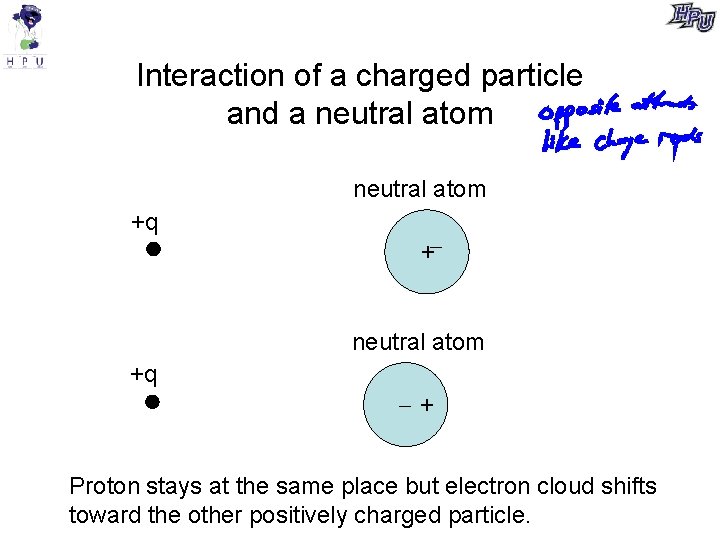
Interaction of a charged particle and a neutral atom +q + Proton stays at the same place but electron cloud shifts toward the other positively charged particle.

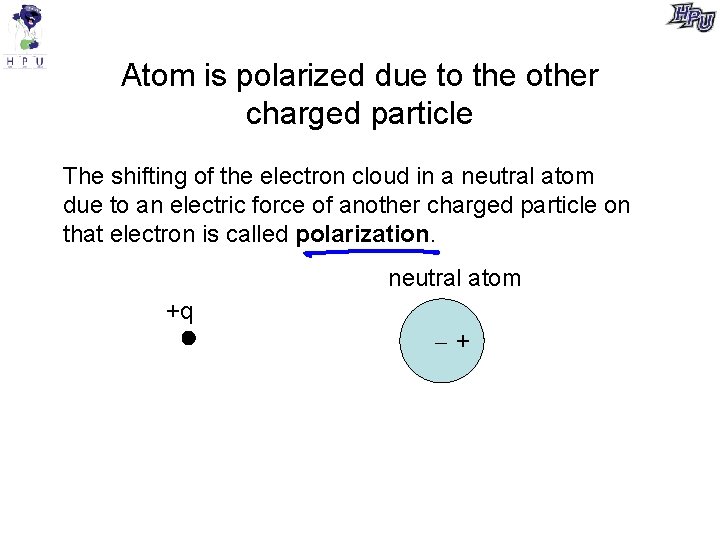
Atom is polarized due to the other charged particle The shifting of the electron cloud in a neutral atom due to an electric force of another charged particle on that electron is called polarization. neutral atom +q +

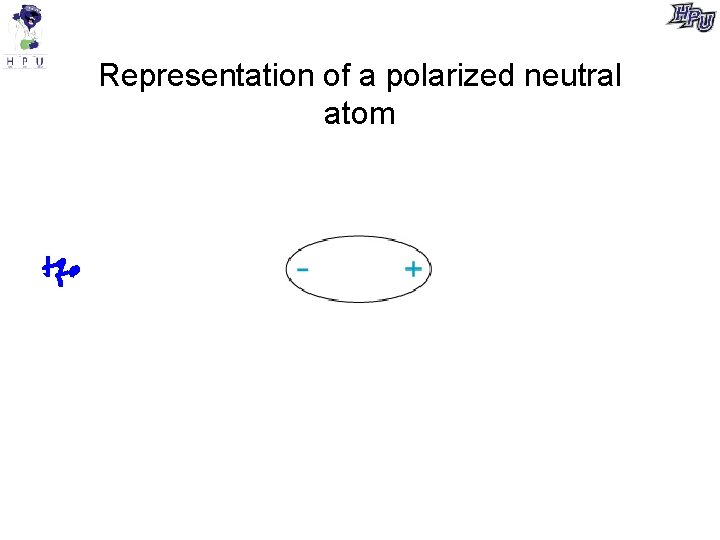
Representation of a polarized neutral atom

Poll Will a charged particle and neutral atom attract, repel, or not interact? 1. attract 2. repel 3. not interact

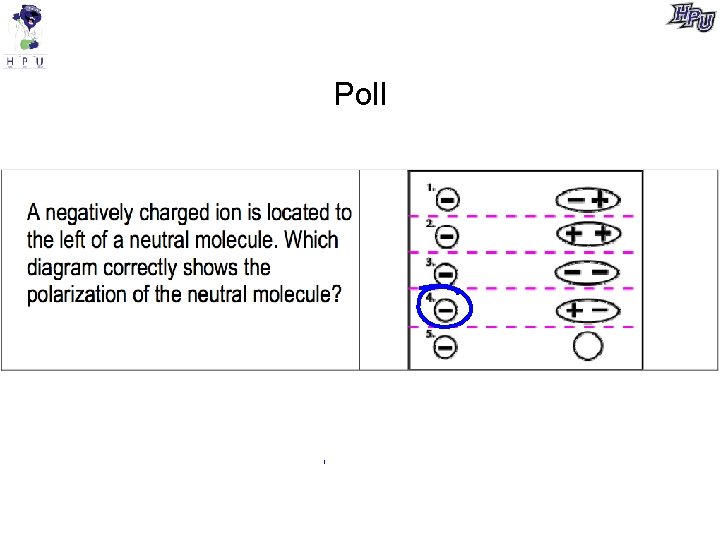
Poll

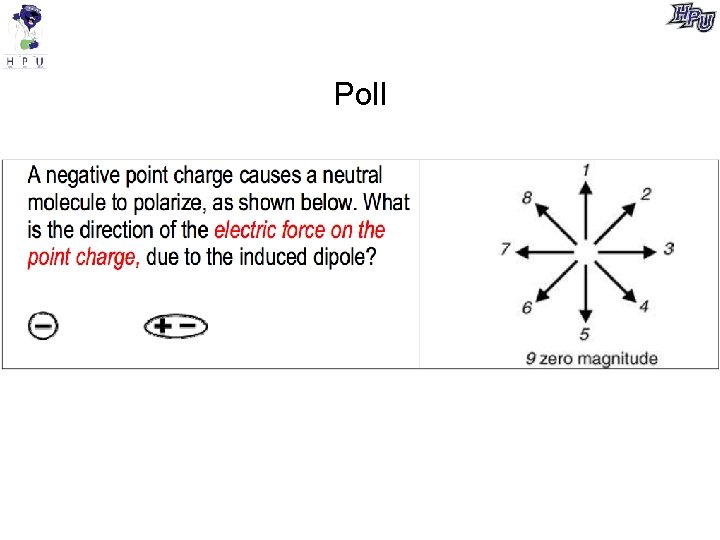
Poll

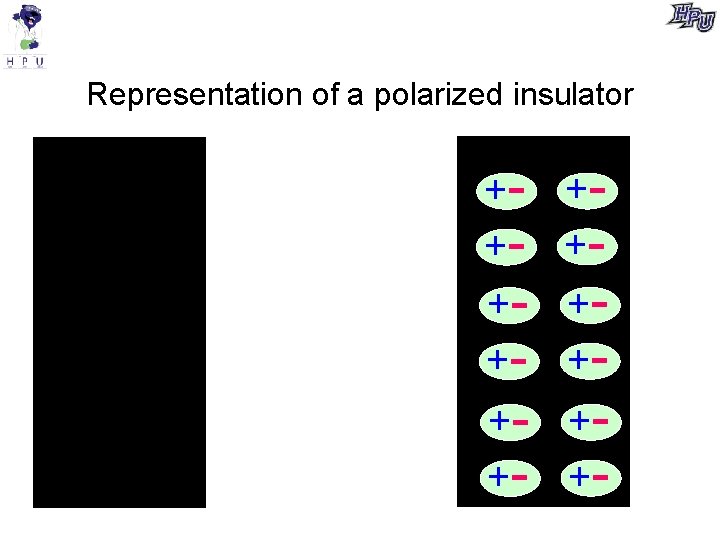
Representation of a polarized insulator ++++- +- +-

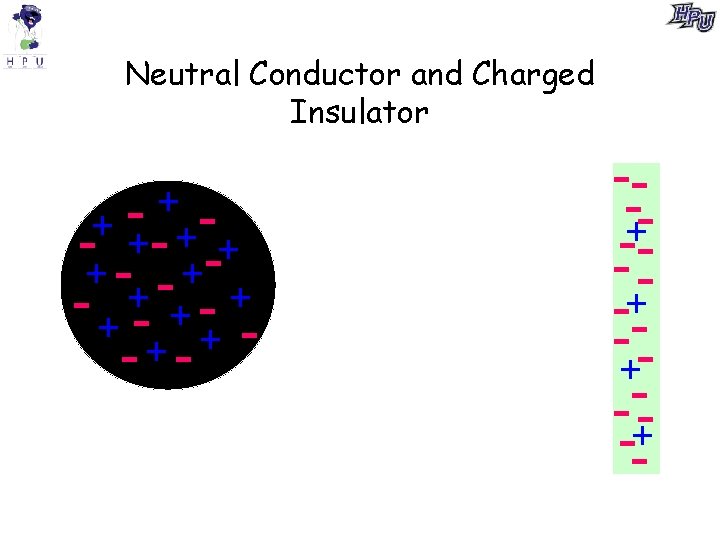
Neutral Conductor and Charged Insulator + + - +- + -+ +- -+ - + +- + + -+- --+-- -+-+ -+--

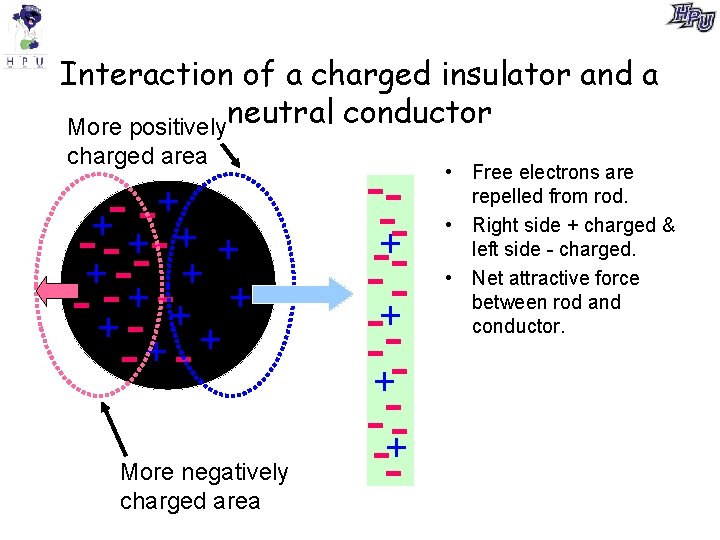
Interaction of a charged insulator and a More positivelyneutral conductor charged area + + - - +-- + + +- + - +- +- -+ + - More negatively charged area --+-- -+-+ -+-- • Free electrons are repelled from rod. • Right side + charged & left side - charged. • Net attractive force between rod and conductor.

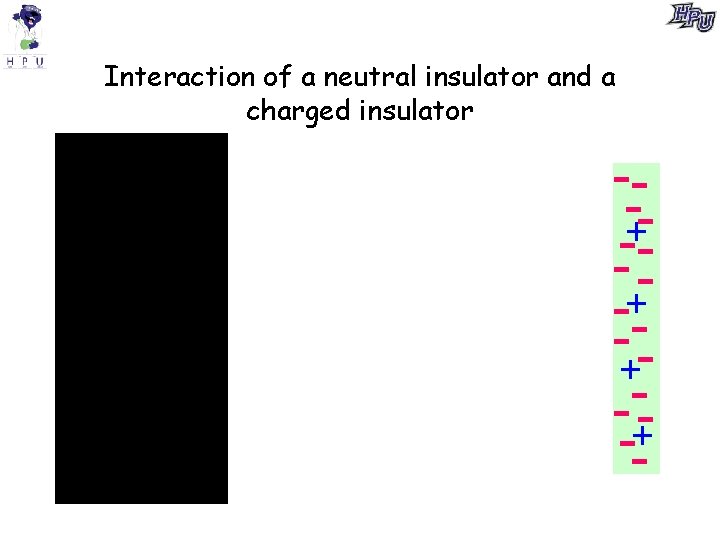
+- +- +- + +- - +- +- Interaction of a neutral insulator and a charged insulator +- --+-- -+-+ -+-- +-

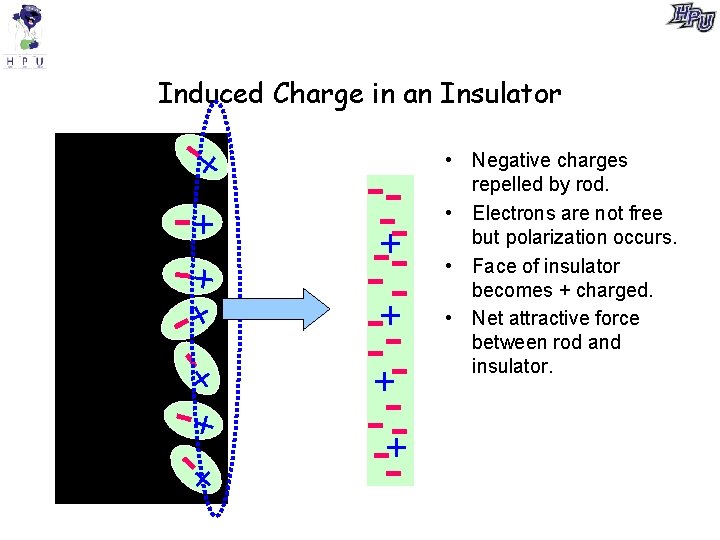
Induced Charge in an Insulator + - +++- + --+-- -+-+ -+-- • Negative charges repelled by rod. • Electrons are not free but polarization occurs. • Face of insulator becomes + charged. • Net attractive force between rod and insulator. +- + -