Cellular Respiration Using food to make energy All



- Slides: 17

Cellular Respiration Using food to make energy All cells do this Occurs in the Mitochondria I. The body uses energy in a form called ATP A. The cell needs ATP to do several different kinds of work 1. Mechanical work (muscles contracting) 2. Transport work (any kind of active transport) 3. Chemical work (enzymes, reactions)

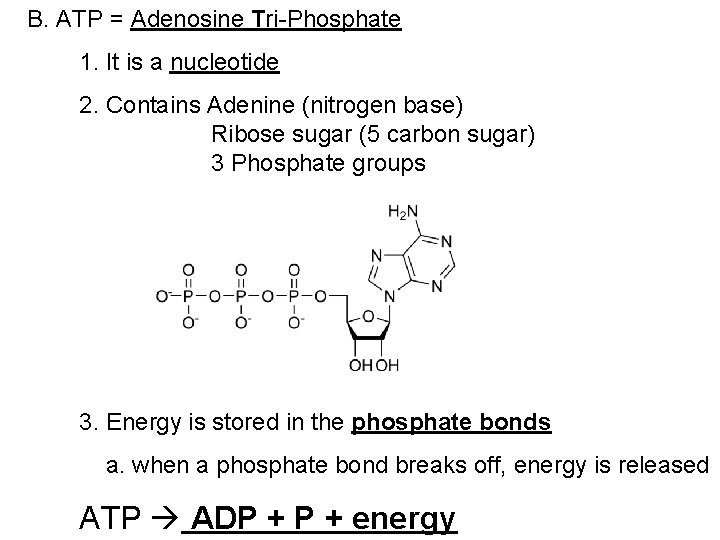
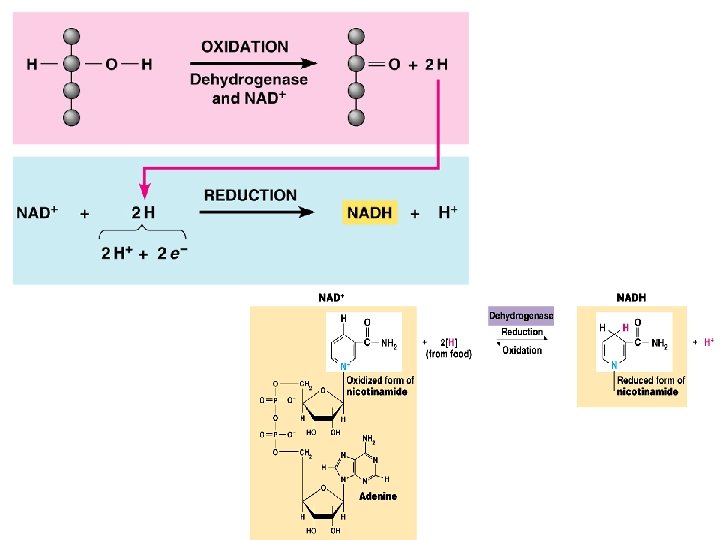
B. ATP = Adenosine Tri-Phosphate 1. It is a nucleotide 2. Contains Adenine (nitrogen base) Ribose sugar (5 carbon sugar) 3 Phosphate groups 3. Energy is stored in the phosphate bonds a. when a phosphate bond breaks off, energy is released ATP ADP + energy

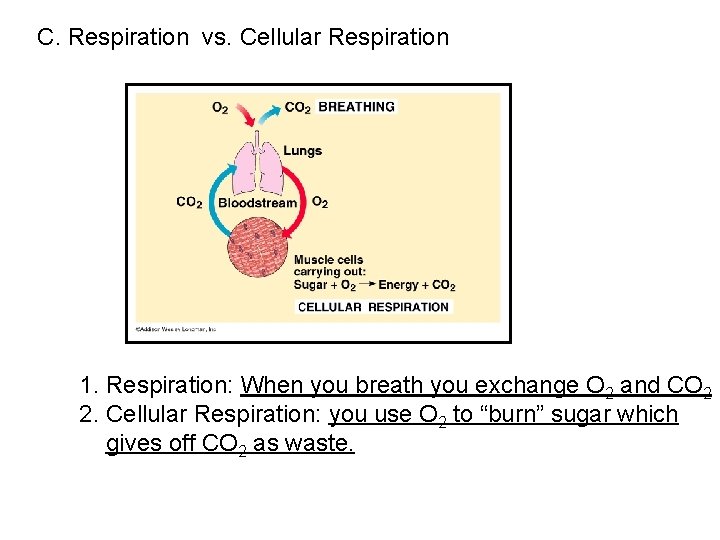
C. Respiration vs. Cellular Respiration 1. Respiration: When you breath you exchange O 2 and CO 2 2. Cellular Respiration: you use O 2 to “burn” sugar which gives off CO 2 as waste.

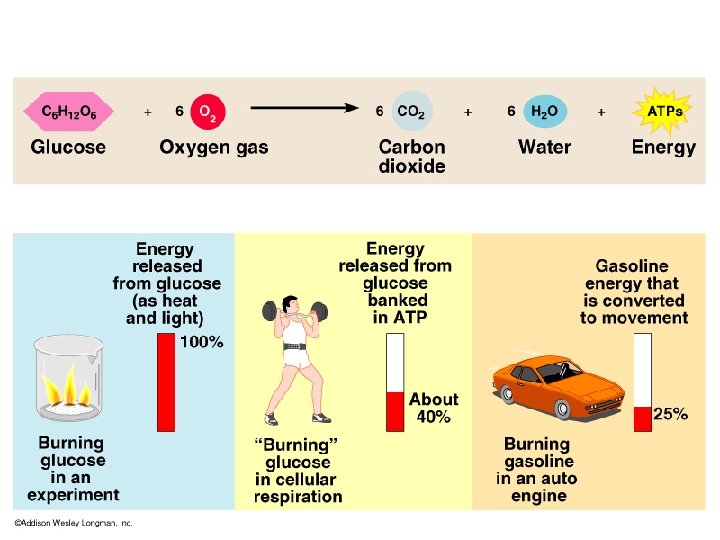
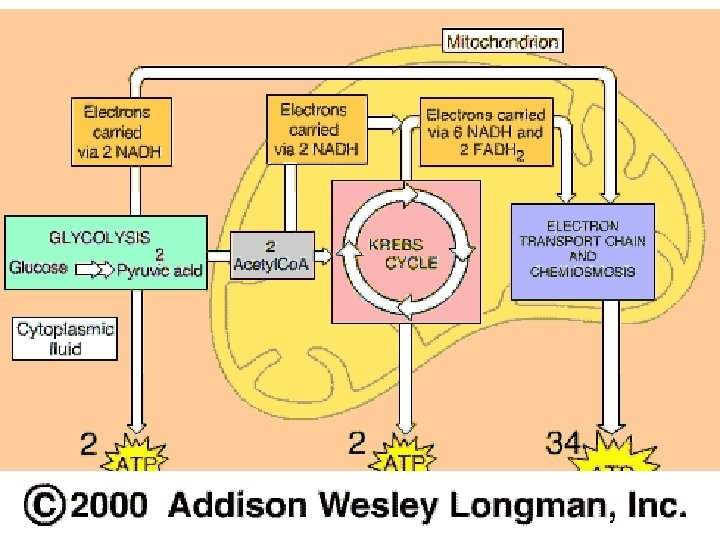
D. General equation of cellular respiration: C 6 H 12 O 6 + 6 O 2 ---> 6 CO 2 + 6 H 2 O + ATP 1. The process is divided into 3 parts: a. Glycolysis = “sugar splitting” b. Krebs cycle = named after Hans Krebs c. Electron Transport Chain 2. Only get 40% of glucose’s potential energy. The rest is converted to heat. 3. Organic molecule is not always glucose (can be fats and protein too)


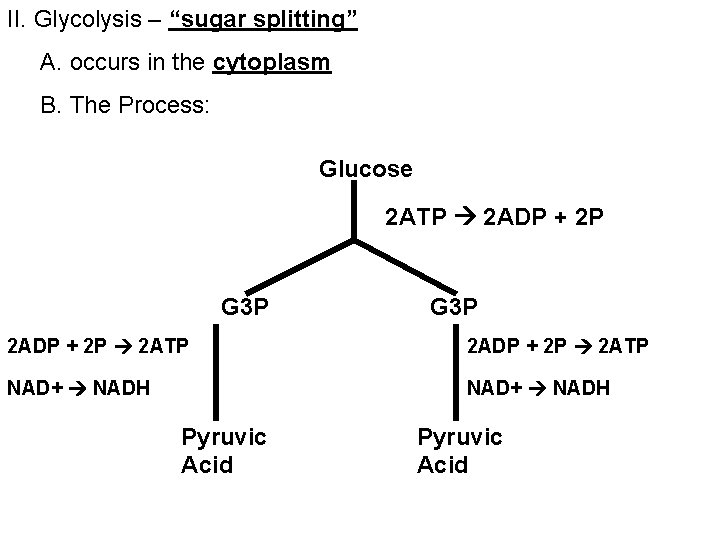
II. Glycolysis – “sugar splitting” A. occurs in the cytoplasm B. The Process: Glucose 2 ATP 2 ADP + 2 P G 3 P 2 ADP + 2 P 2 ATP NAD+ NADH Pyruvic Acid

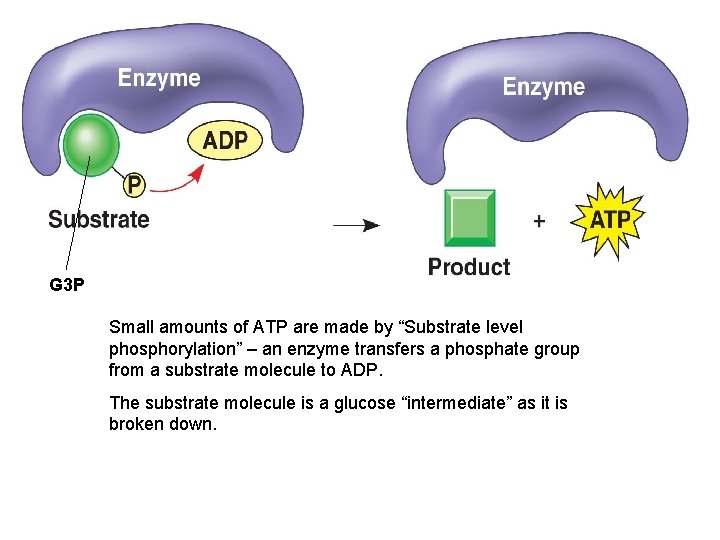
G 3 P Small amounts of ATP are made by “Substrate level phosphorylation” – an enzyme transfers a phosphate group from a substrate molecule to ADP. The substrate molecule is a glucose “intermediate” as it is broken down.


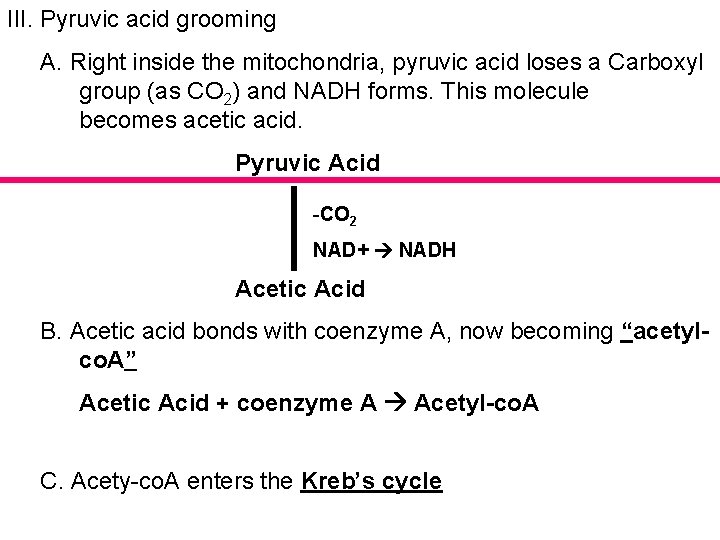
III. Pyruvic acid grooming A. Right inside the mitochondria, pyruvic acid loses a Carboxyl group (as CO 2) and NADH forms. This molecule becomes acetic acid. Pyruvic Acid -CO 2 NAD+ NADH Acetic Acid B. Acetic acid bonds with coenzyme A, now becoming “acetylco. A” Acetic Acid + coenzyme A Acetyl-co. A C. Acety-co. A enters the Kreb’s cycle

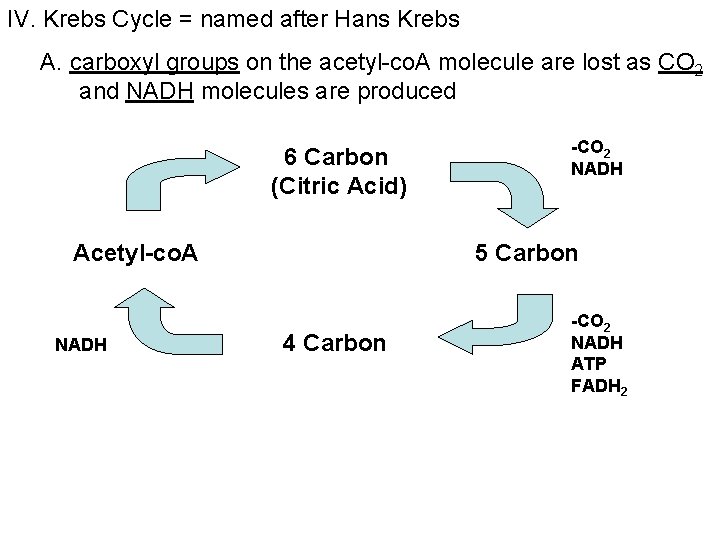
IV. Krebs Cycle = named after Hans Krebs A. carboxyl groups on the acetyl-co. A molecule are lost as CO 2 and NADH molecules are produced 6 Carbon (Citric Acid) Acetyl-co. A NADH -CO 2 NADH 5 Carbon 4 Carbon -CO 2 NADH ATP FADH 2

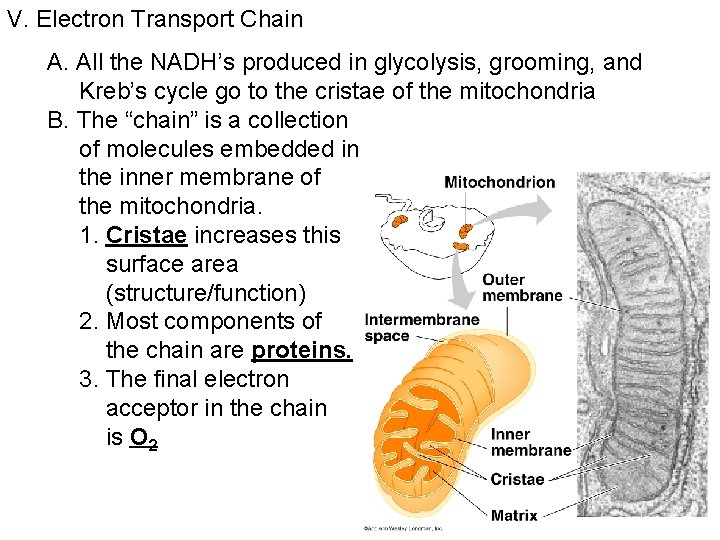
V. Electron Transport Chain A. All the NADH’s produced in glycolysis, grooming, and Kreb’s cycle go to the cristae of the mitochondria B. The “chain” is a collection of molecules embedded in the inner membrane of the mitochondria. 1. Cristae increases this surface area (structure/function) 2. Most components of the chain are proteins. 3. The final electron acceptor in the chain is O 2

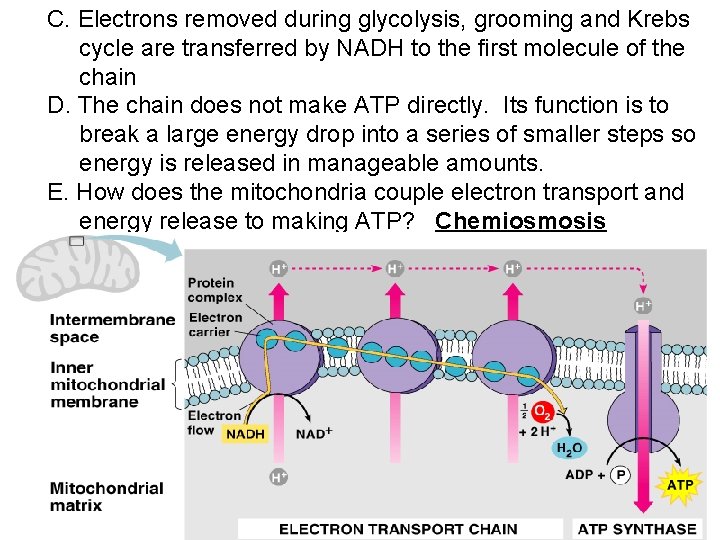
C. Electrons removed during glycolysis, grooming and Krebs cycle are transferred by NADH to the first molecule of the chain D. The chain does not make ATP directly. Its function is to break a large energy drop into a series of smaller steps so energy is released in manageable amounts. E. How does the mitochondria couple electron transport and energy release to making ATP? Chemiosmosis

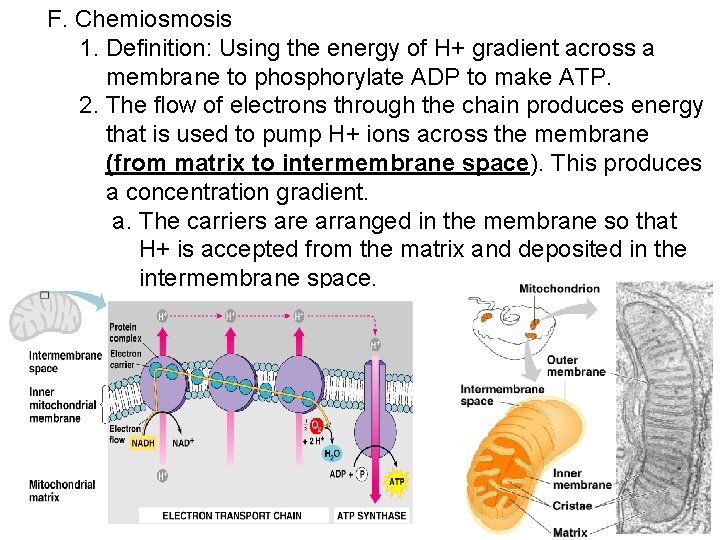
F. Chemiosmosis 1. Definition: Using the energy of H+ gradient across a membrane to phosphorylate ADP to make ATP. 2. The flow of electrons through the chain produces energy that is used to pump H+ ions across the membrane (from matrix to intermembrane space). This produces a concentration gradient. a. The carriers are arranged in the membrane so that H+ is accepted from the matrix and deposited in the intermembrane space.

3. How ATP is made from this gradient: a. ATP Synthase is the enzyme that makes ATP. It is located in the inner membrane of the mitochondria. Made of three main parts: a clinder within the inner mitochondrial membrane a knob protruding into the mitochondrial matrix an internal rod connecting the two. b. ATP Synthase uses the energy from the gradient to make ATP. c. When H+ ions flow through the cylinder, with their gradient, they cause the cylinder and the attached rod to rotate. d. The spinning rod causes the knob to change shape. This activates sites on the enzyme to bond ADP and P to make ATP. e. For every NADH that enters the ETC, 3 ATPs result f. For every FADH 2 that enters the ETC, 2 ATPs result


VI. Anaerobic Respiration – When oxygen is NOT available There are 2 chemical pathways depending on the type of cell. A. Lactic Acid Fermentation 1. Starts with Glycolysis 2. Pyruvic acid Lactic Acid a. no additional ATP is formed b. muscles cramp, burn, sore due to lactic acid. 3. Food spoilage (botulism) 4. use process to make yogurt and sauerkraut

B. Alcoholic Fermentation 1. Starts with glycolysis 2. Pyruvic acid Ethyl alcohol + CO 2 a. no additional ATP is made 3. Use process to make wine and beer 4. yeast cells go through this process, bread rises. VII. Differences A. Fermentation = Anaerobic Respiration =Aerobic B. Fermentation final e- acceptor= pyruvic acid Respiration final e- acceptor = oxygen C. Fermentation generates 2 ATP Respiration generates 34 --38 ATP