Argon Ar Science per 6 Ms Caswell 11907










- Slides: 12

Argon Ar Science per. 6 Ms. Caswell 11/9/07

My Element: Argon www. google. com

• Symbol-Ar • 18 Protons and Electrons • 22 Neutrons • Atomic Number-18 • Atomic Mass- 39. 948 amu • melting point-(-189. 3 dergrees C) • boiling point-(-186 degrees C) • Crystal Structure- Cubic • Color- Colorless gas • Classification- Noble Gas • Group 18 - 8 valence electrons • period 3 - 3 energy levels Google images

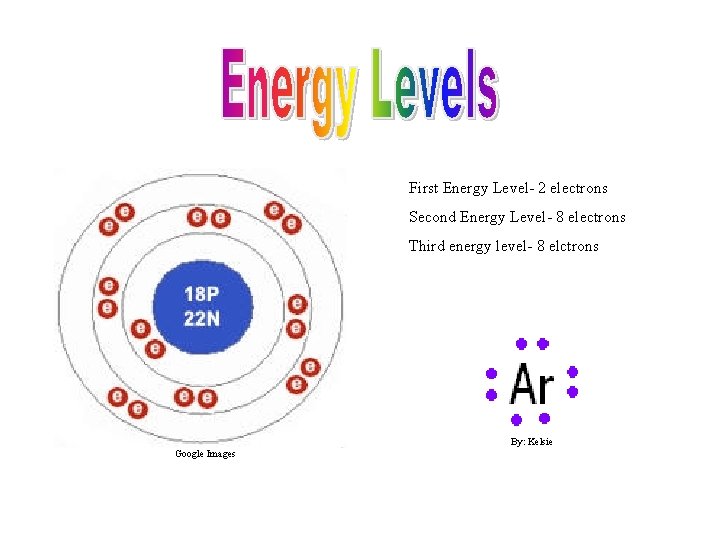
First Energy Level- 2 electrons Second Energy Level- 8 electrons Third energy level- 8 elctrons By: Kelsie Google Images

History Argon was discovered in Scotland in 1894 by Lord Rayleigh and Sir William Ramsay. They discovered it by removing Nitrogen, Oxygen, Carbon Dioxide and Water from Liquid Air. All that was left was Argon.

Argon was discovered in natural gases and some minerals. It was the first inert gas to be discovered. It impacted history because we found a new way to use flourescent lighting because we are running out of tungsten.

• Physical Properties. Odorless Colorless gas Tasteless Gas at room temperature 27 degrees C Not a metal, metalloid, or nonmetal Chemical Properties. Soluble www. arroyoseco. org Argon is not found in any compounds.

Compounds and Uses of Argon is not found in any compounds. Uses- fluorescent lighting light bulbs lamp gas lasers welding

A common use is fire suppression for sensitive computer and or electrical equipment. “I hate Argon! I can’t breath!!”

Presentation Answers • • • Argon is not known to have any positive or negative effects on living things. The only hazard of Argon is Asphyxia which is when Argon takes the place of the air you breath and you suffocate. Argon can not be recycled and it is not a hazard to the environment. www. tesladownunder. iinet. au

There is a large building in Dallas Texas that as lighted by Argon because Neon does not have a green color. Argon is heavier than air. When electricity passes through Argon, it glows in a pale red color. Part of Mars’ atmosphere is made of Argon. 1% of the Earth’s atmosphere is made up of Argon is not an active substance in living things. Argon is also found in the Earth’s crust. Argon is chemically inactive. On rare occasions, it forms weak, compound-like structures. http: //gilglover. com/argon 2. jpg

Bibliography • • • Websites www. chemicalelements. com www. webelements. com www. google. com www. ask. com • Guide to the Elements By: Albert Stwertka Copyright year- 1998 • Actinium to Fluorine By: Grolier Educational • Books • Chemical Elements By: David E. Newton • World Book Copyright year- 2002