Aquatic Chemistry 367 Civil and Environmental Engineering Meeting








- Slides: 11

Aquatic Chemistry 367 Civil and Environmental Engineering Meeting time: MWF 11: 00 -11: 50 am Meeting room: Abbott Auditorium in Pancoe Pavillion Instructor: Jean-Francois Gaillard, jfgaillard@northwestern. edu Grader: Amy Dahl, a-dahl@northwestern. edu

Logistics • Introductions • http: //www. civil. northwestern. edu/ehe/cour ses/ce-367. htm • Keep on top of the homework!

Why study Aquatic Chemistry?

Why study Aquatic Chemistry? • Required for life • Pollution transport almost always requires water • Many environmentally relevant reactions occur in water • Critical for controlling cycling of many elements – Nutrients – Toxins – Oxygen, nitrogen, carbon dioxide – atmosphere control

Applications of aquatic chemistry: • • • Arsenic Chromium Mercury Acid mine drainage Global warming

What is water? • • • Universal Solvent Required for life Molecular structure Hydrogen bonding: water clustering Anomalous behavior compared to other liquids

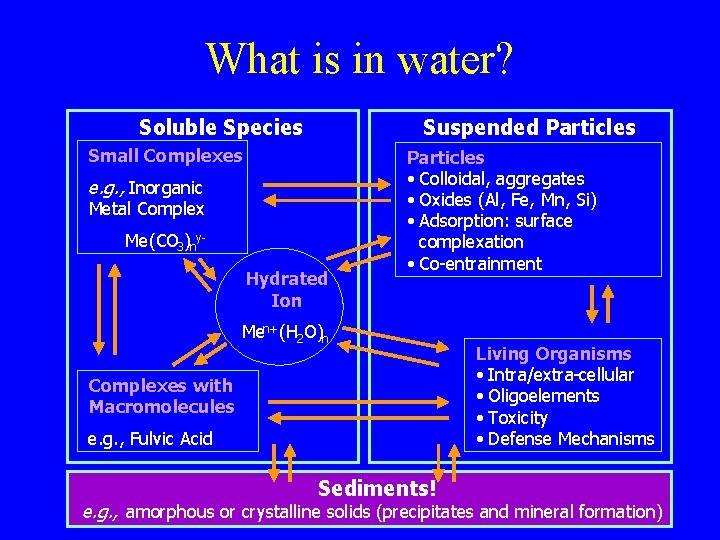
What is in water? Soluble Species Suspended Particles Small Complexes e. g. , Inorganic Metal Complex Me(CO 3 )ny. Hydrated Ion Particles • Colloidal, aggregates • Oxides (Al, Fe, Mn, Si) • Adsorption: surface complexation • Co-entrainment Men+ (H 2 O)n Complexes with Macromolecules e. g. , Fulvic Acid Sediments! Sediments Living Organisms • Intra/extra-cellular • Oligoelements • Toxicity • Defense Mechanisms e. g. , amorphous ore. g. , crystalline solidsor(precipitates and mineral formation) amorphous crystalline solids

What is in water? • Dissolved ions: – – – Major Cations (Ca, Na, Mg, K, Fe) Trace elements (Zn, Cr, Cu, Cd) Anions (F, Cl, nitrate, sulfate) Complexes (non-charged) Organic molecules (humic substances) • http: //www. ar. wroc. pl/~weber/kwasy 2. htm • Particulate – Organic matter – Clays – Oxides

Chemical Reactions • • Acid-base chemistry Coordination chemistry Precipitation and dissolution Redox reactions

Kinetic approach • Does not assume equilibrium has been reached • Use a rate constant, k, to describe the rate of formation of products or consumption of reactants

Thermodynamic explanation • Assumes equilibrium of a reaction is reached • Simplifies solving mathematical expressions of chemical systems • Use an equilibrium constant, K, to describe ratio of products to reactants
 Whitely v chappel 1868
Whitely v chappel 1868 Magor and st mellons v newport corporation
Magor and st mellons v newport corporation R v allen 1872
R v allen 1872 Cmput 367
Cmput 367 R v allen (1872) lr 1 ccr 367
R v allen (1872) lr 1 ccr 367 Cs 367
Cs 367 Round off to the nearest ten thousands
Round off to the nearest ten thousands Direct instruction strategies
Direct instruction strategies Cs 367
Cs 367 Ap environmental science biodiversity
Ap environmental science biodiversity Civil rights and civil liberties webquest
Civil rights and civil liberties webquest What is meeting and types of meeting
What is meeting and types of meeting