Macromolecular Chemistry Lecture 10 Chemistry 367 L392 N




- Slides: 18

Macromolecular Chemistry Lecture 10 Chemistry 367 L/392 N

Midterm Exam l l l l Where: Right here…. in the lecture room WEL 3. 502 When: Next Tuesday 2/24/09 at 3: 30 – 5 PM What: Covers lectures through Thursday 2/18 Bring: Pencil, eraser, Calculator only…closed book! Do: Study lecture notes, homework, reading assignments and graduate presentations Do not: Memorize the free radical kinetics equations…but do know the principles! Go over homework problems. Please: Do a good job! Chemistry 367 L/392 N

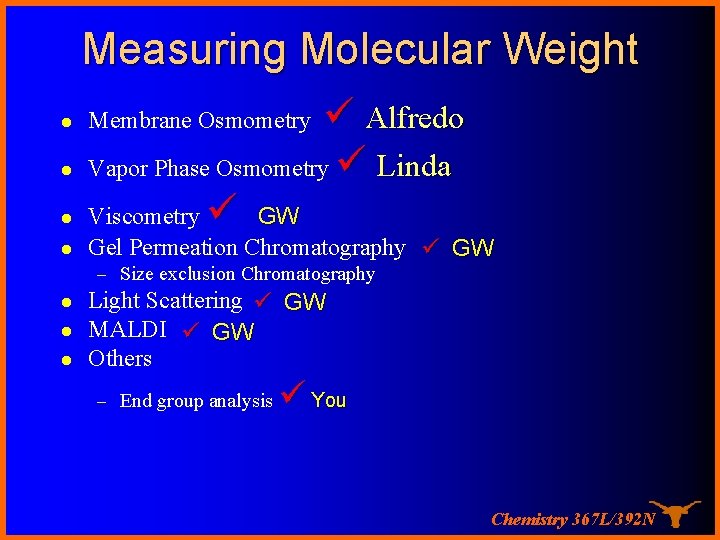
Measuring Molecular Weight l Alfredo Vapor Phase Osmometry Linda Viscometry GW l Gel Permeation Chromatography GW l l Membrane Osmometry – Size exclusion Chromatography l l l Light Scattering GW MALDI GW Others – End group analysis You Chemistry 367 L/392 N

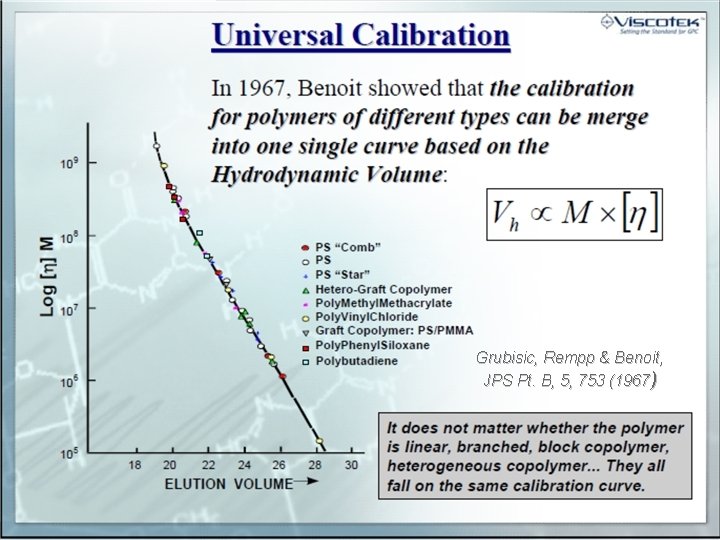
Setting the Standard for GPC. Grubisic, Rempp & Benoit, JPS Pt. B, 5, 753 (1967)

Setting the Standard for GPC.

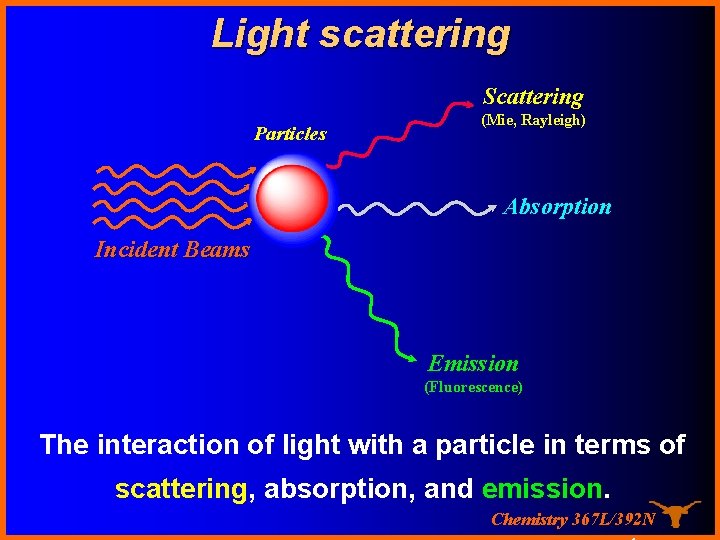
Light scattering Scattering Particles (Mie, Rayleigh) Absorption Incident Beams Emission (Fluorescence) The interaction of light with a particle in terms of scattering, absorption, and emission. Chemistry 367 L/392 N

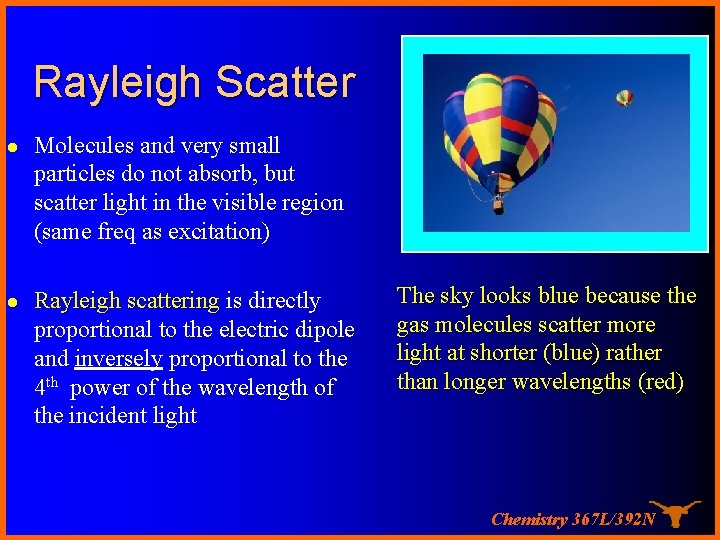
Rayleigh Scatter l Molecules and very small particles do not absorb, but scatter light in the visible region (same freq as excitation) l Rayleigh scattering is directly proportional to the electric dipole and inversely proportional to the 4 th power of the wavelength of the incident light The sky looks blue because the gas molecules scatter more light at shorter (blue) rather than longer wavelengths (red) Chemistry 367 L/392 N

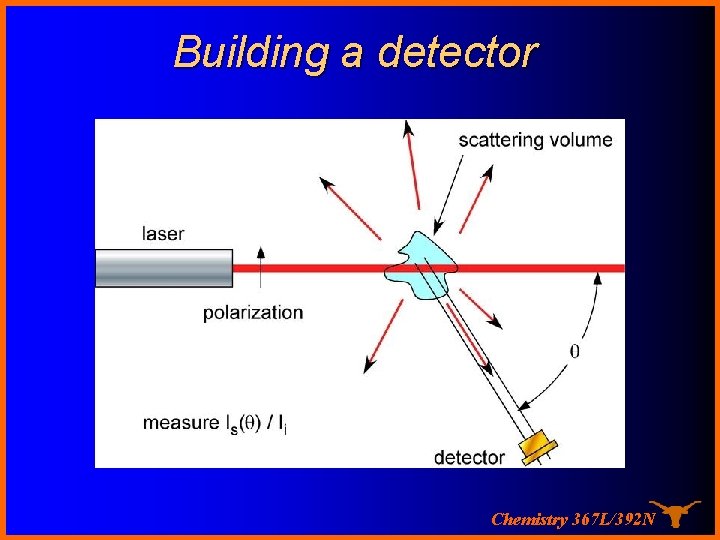
Building a detector In the lab… Chemistry 367 L/392 N

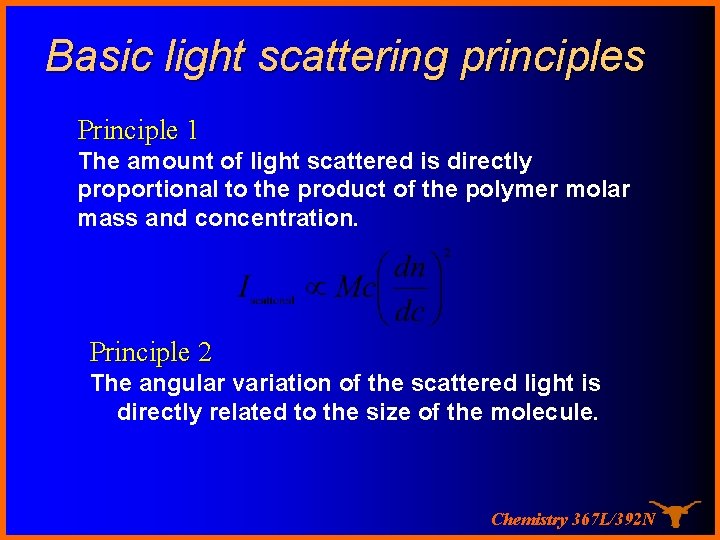
Basic light scattering principles Principle 1 The amount of light scattered is directly proportional to the product of the polymer molar mass and concentration. Principle 2 The angular variation of the scattered light is directly related to the size of the molecule. Chemistry 367 L/392 N

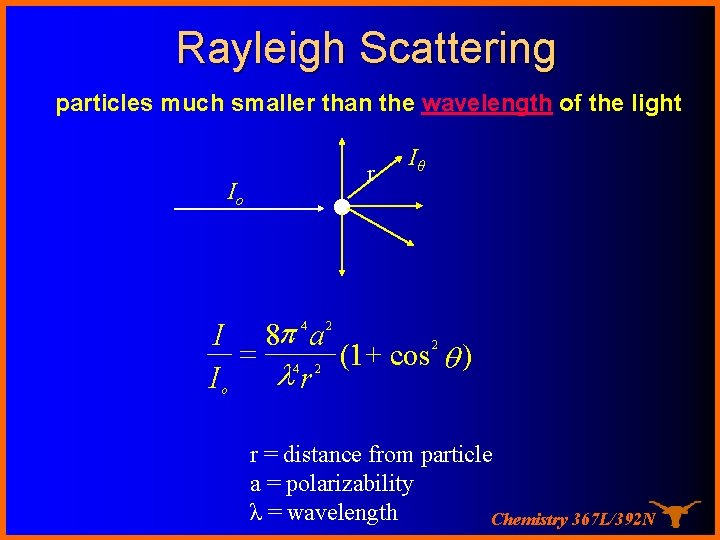
Rayleigh Scattering particles much smaller than the wavelength of the light Io ● r Iθ I 8 p a = (1 + cos q ) lr I 4 2 2 4 2 o r = distance from particle a = polarizability λ = wavelength Chemistry 367 L/392 N

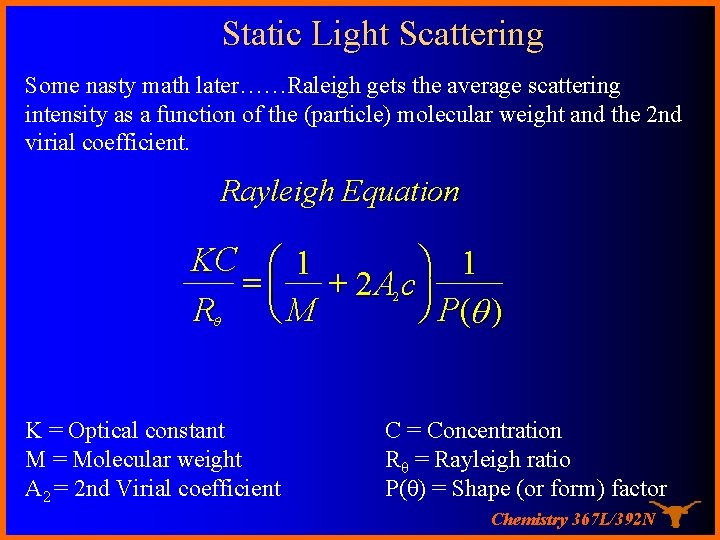
Static Light Scattering Some nasty math later……Raleigh gets the average scattering intensity as a function of the (particle) molecular weight and the 2 nd virial coefficient. Rayleigh Equation KC æ 1 ö 1 = ç + 2 A c÷ ø P(q ) Rq è M 2 K = Optical constant M = Molecular weight A 2 = 2 nd Virial coefficient C = Concentration Rθ = Rayleigh ratio P(θ) = Shape (or form) factor Chemistry 367 L/392 N

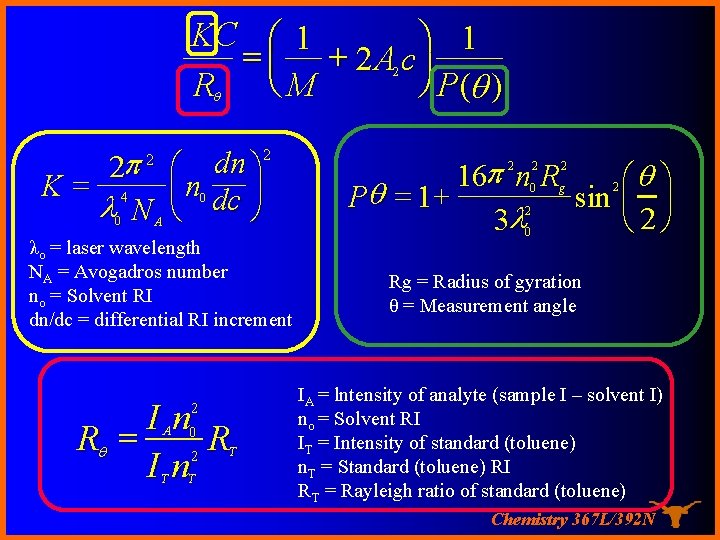
KC æ 1 ö 1 = ç + 2 A c÷ ø P(q ) Rq è M 2 2 p K= 4 l N 2 2 0 æ dn ö ç n dc ÷ è ø 0 A λo = laser wavelength NA = Avogadros number no = Solvent RI dn/dc = differential RI increment I n 0 Rq = R 2 In 2 A T T T 16 p n R æq ö Pq = 1 + sin ç ÷ è 2ø 3 l 2 2 2 0 g 2 2 0 Rg = Radius of gyration θ = Measurement angle IA = lntensity of analyte (sample I – solvent I) no = Solvent RI IT = Intensity of standard (toluene) n. T = Standard (toluene) RI RT = Rayleigh ratio of standard (toluene) Chemistry 367 L/392 N

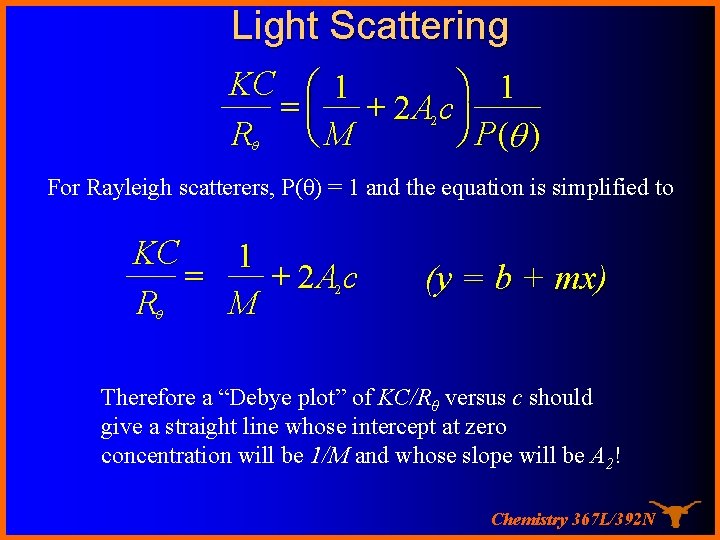
Light Scattering KC æ 1 ö 1 = ç + 2 A c÷ ø P(q ) Rq è M 2 For Rayleigh scatterers, P(θ) = 1 and the equation is simplified to KC 1 = + 2 A c Rq M 2 (y = b + mx) Therefore a “Debye plot” of KC/Rθ versus c should give a straight line whose intercept at zero concentration will be 1/M and whose slope will be A 2! Chemistry 367 L/392 N

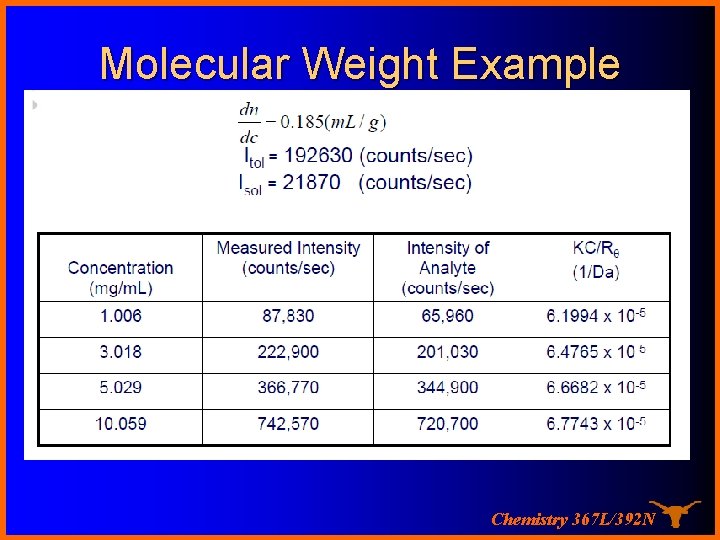
Molecular Weight Example Chemistry 367 L/392 N

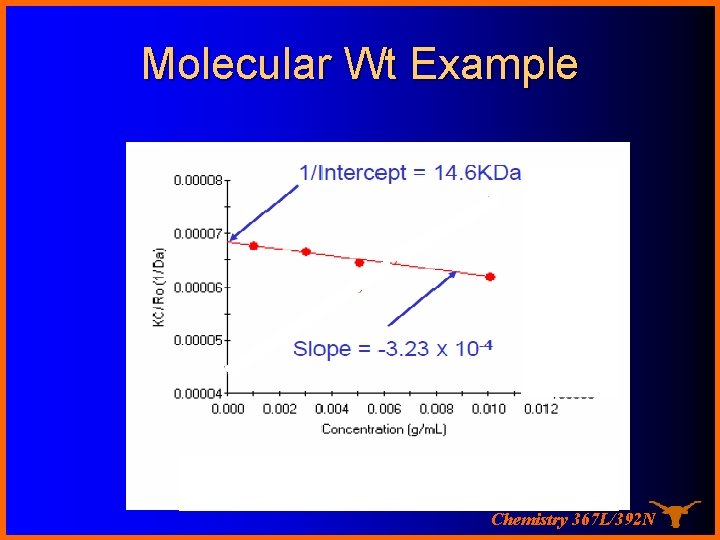
Molecular Wt Example Chemistry 367 L/392 N

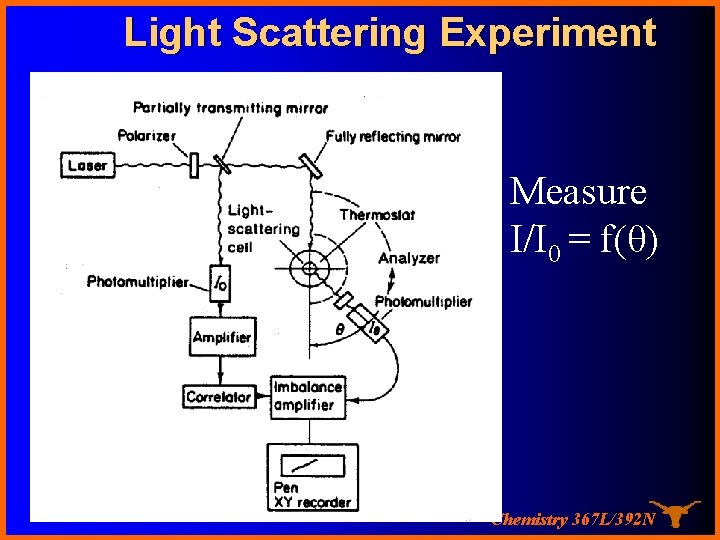
Light Scattering Experiment Measure I/I 0 = f(θ) Chemistry 367 L/392 N

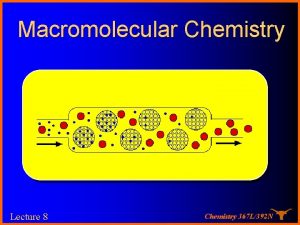
For SEC l l l This concept has been embodied in detectors These measurements give an absolute value for Mw Remember that there are some assumptions made that limit the utility of the “simple” detectors to Molecular weights below abut 150, 000 Daltons dn/dc can be measured with an RI detector A “significant” difference in the refractive index of the sample and the solvent is required for good signal. Chemistry 367 L/392 N

Study Hard Chemistry 367 L/392 N
 Purposive approach
Purposive approach 367 562 rounded to the nearest 100 000
367 562 rounded to the nearest 100 000 Cmput 367
Cmput 367 Indirect instruction definition
Indirect instruction definition Whitely v chappel
Whitely v chappel R v allen (1872) lr 1 ccr 367
R v allen (1872) lr 1 ccr 367 Cs 367
Cs 367 Advantages and disadvantages of statutory interpretation
Advantages and disadvantages of statutory interpretation Cs367
Cs367 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Inorganic vs organic chemistry
Inorganic vs organic chemistry Functional groups ib chemistry
Functional groups ib chemistry Www.imran lecture.com
Www.imran lecture.com Peter zumthor lecture
Peter zumthor lecture Performance management lecture
Performance management lecture Reading goals
Reading goals Waves notes pdf download
Waves notes pdf download