Applied Physics and Chemistry Gases Lecture 1 Gases





- Slides: 8

Applied Physics and Chemistry Gases Lecture 1

Gases � Properties: › › No definite shape No definite volume Easily compressed Mixes completely with any other gas

Pressure � Kinetic molecular theory: › All particles of matter are in constant motion › Particles of a gas move fast and hit the container � Pressure: › Force of collisions of gas particles over an area of the container

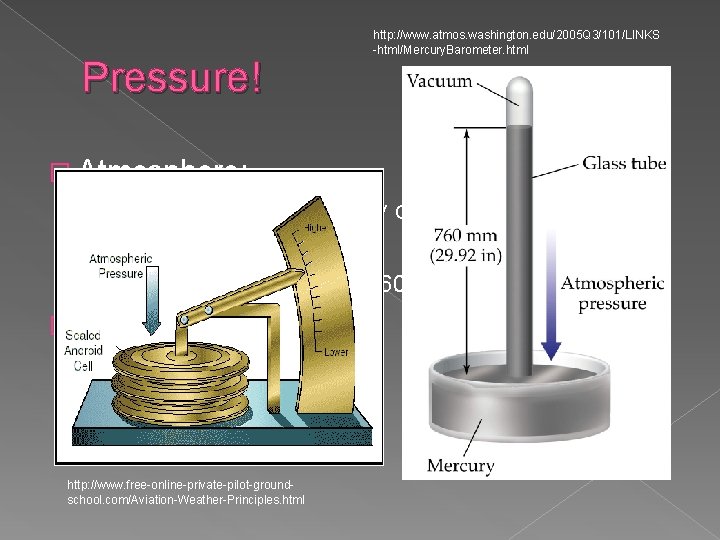
Pressure! http: //www. atmos. washington. edu/2005 Q 3/101/LINKS -html/Mercury. Barometer. html � Atmosphere: › Due to the pull of gravity on gases › Measured by barometer �Evangelista Torricelli (1608 -1647) � Measuring: › Mercury barometer › Anaeroid barometer http: //www. free-online-private-pilot-groundschool. com/Aviation-Weather-Principles. html

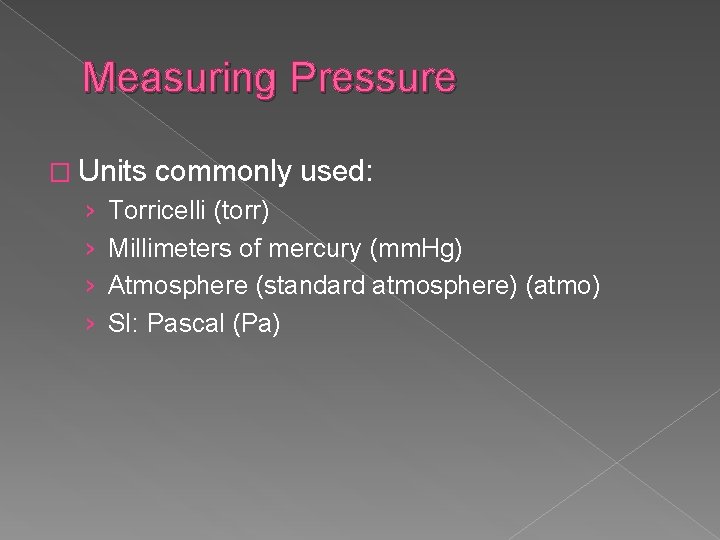
Measuring Pressure � Units › › commonly used: Torricelli (torr) Millimeters of mercury (mm. Hg) Atmosphere (standard atmosphere) (atmo) SI: Pascal (Pa)

Pressure conversions � 1 › › › atmosphere = 760 mm Hg 760 torr 101 325 Pa 101. 325 k. Pa 14. 69 psi (ACK!) � Use conversion factors (carefully showing ALL WORK) to convert from one unit to another!

Example of conversion! � The pressure of air in a tire is recorded as 192 000 Pa. What is this pressure in atmospheres? � What we know: › P = 192 000 Pa � Conversion factors: › 1 atmo = 101 325 Pa › 192 000 Pa x 1 atmo = › 101 325 Pa 1. 895 atmo

Another Example: On a summer day in Breckenridge CO, the atmospheric pressure is 525 mm Hg. What is this pressure in atmospheres? � What we know: � › P = 525 mm Hg � Conversion factor: › 1 atmo = 760 mm Hg � Do the math! › 525 mm Hg x 1 atmo = 0. 69 atmo › 760 mm Hg