Allocation systems in liver transplantation Farzad Kakaei MD










- Slides: 16

Allocation systems in liver transplantation Farzad Kakaei, MD, Associate professor of surgery, Tabriz University of medical sciences, Tabriz, Iran

Introduction • Approaches to the problem of graft scarcity, • Increasing donor pool (living, brain death, cardiac death) • Improving alternative treatments for liver diseases and hepatocellular cancer. • Liver allocation policies • The goal of allocation systems : the best possible outcomes for the waiting list • We need for change in our policy because of : • Changes in the etiology of cirrhosis over time • Recent expansion of indications for liver transplantation (including cholangiocarcinoma and colorectal liver metastases) • Geopolitical and social environments • Expansion of liver transplant centers

A brief history • Procurement and allocation of donor organs was performed locally by individual hospital-based teams in the early era of liver transplantation • Reducing the mortality rate to less than 10% by 1984 results in a series of high profile controversial transplants with accusations of bias, arbitrary decision-making and manipulation of transplant waiting lists in return for financial donations • National Organ Transplant Act in 1984 (criminalizing financial compensation for donor organs). • Regional networks for organ procurement and distribution were established with the aim of transferring the responsibility for organ allocation from individual physicians to evidence-based, objective and transparent algorithms based primarily on clinical need • Similar fundamental principles were subsequently adopted by all countries and centres.

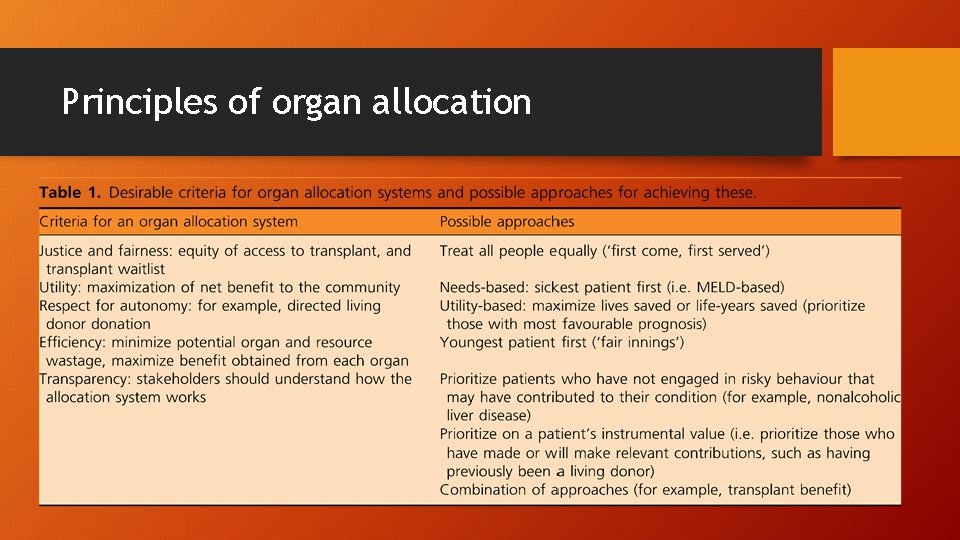
Principles of organ allocation

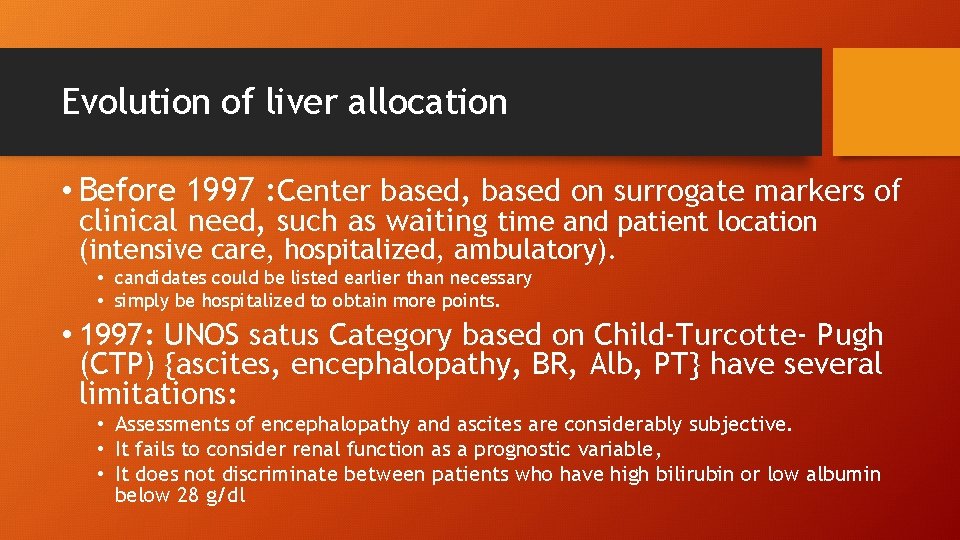
Evolution of liver allocation • Before 1997 : Center based, based on surrogate markers of clinical need, such as waiting time and patient location (intensive care, hospitalized, ambulatory). • candidates could be listed earlier than necessary • simply be hospitalized to obtain more points. • 1997: UNOS satus Category based on Child-Turcotte- Pugh (CTP) {ascites, encephalopathy, BR, Alb, PT} have several limitations: • Assessments of encephalopathy and ascites are considerably subjective. • It fails to consider renal function as a prognostic variable, • It does not discriminate between patients who have high bilirubin or low albumin below 28 g/dl



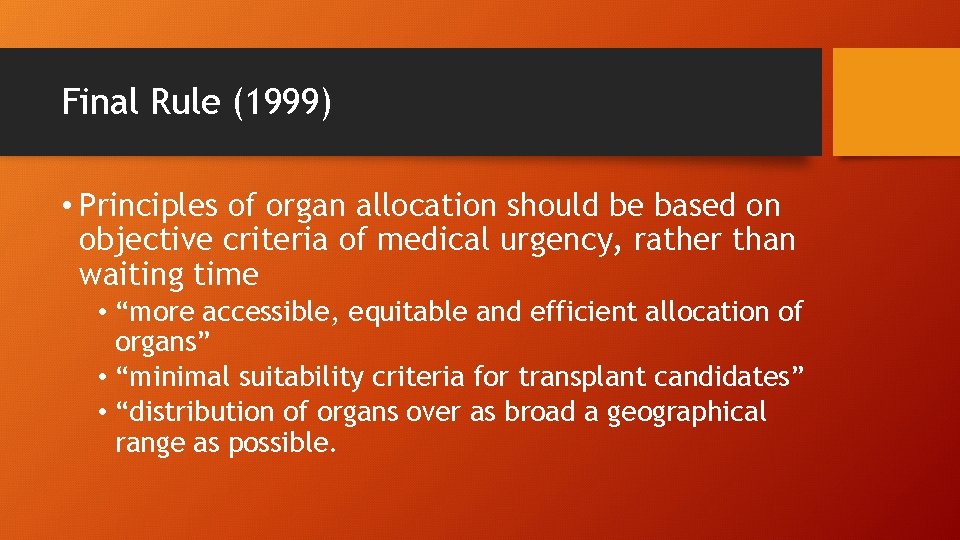
Final Rule (1999) • Principles of organ allocation should be based on objective criteria of medical urgency, rather than waiting time • “more accessible, equitable and efficient allocation of organs” • “minimal suitability criteria for transplant candidates” • “distribution of organs over as broad a geographical range as possible.

The MELD era (February 2002) • Initially developed for survival prediction after TIPS. • Based on bilirubin, creatinine and INR • Introduced to the US in 2002 and used by many countries since. • Reduction in waiting list mortality • small overall increase in early post-transplant mortality • Failure to account for elevations in INR and creatinine for reasons other than liver disease or inaccurate laboratory results • Failure to include other problems such as pruritus, ascites, GIB, HCC, polycystic liver disease, Caroli syndrome, bile duct injury, liver trauma and acute liver failure. • Systematically biased against women • No space for donor-recipient matching

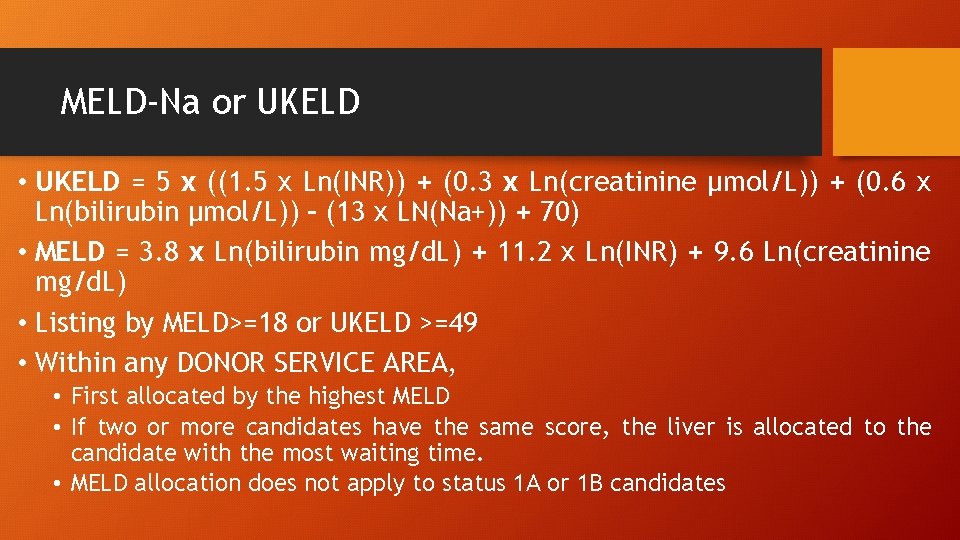
MELD-Na or UKELD • UKELD = 5 x ((1. 5 x Ln(INR)) + (0. 3 x Ln(creatinine μmol/L)) + (0. 6 x Ln(bilirubin μmol/L)) – (13 x LN(Na+)) + 70) • MELD = 3. 8 x Ln(bilirubin mg/d. L) + 11. 2 x Ln(INR) + 9. 6 Ln(creatinine mg/d. L) • Listing by MELD>=18 or UKELD >=49 • Within any DONOR SERVICE AREA, • First allocated by the highest MELD • If two or more candidates have the same score, the liver is allocated to the candidate with the most waiting time. • MELD allocation does not apply to status 1 A or 1 B candidates

MELD exceptions • • • UNOS STATUS 1 (acute hepatic failure) HCC HEPATOPULMONARY SYNDROME (Pa. O 2<60 with a confirmed shunt) PORTOPULMONARY SYNDROME (25<PAP<35 mm. Hg) CYSTIC FIBROSIS FAMILIAL AMYLOID SYNDROME PRIMARY HYPEROXALURIA HILAR CHOLANGIOCARCINOMA FAMILIAL HYPERCHOLESTROLEMIA, Polycystic liver disease, … (? )

UK status prior to 2018 • Allocation on a regional basis • Priority given to the transplant center assigned to a designated organ recovery zone. • Individual waiting list • Compatibility (size and blood group) • UKELD • Logistical benefits • Minimizing graft cold ischemic time, • Perpetuate some ongoing geographical inequity in organ allocation.

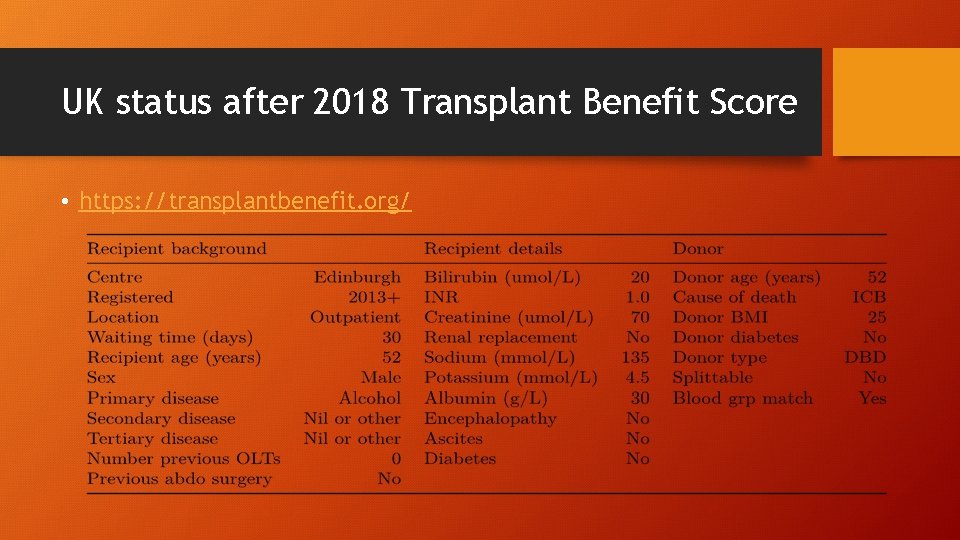
UK status after 2018 Transplant Benefit Score • https: //transplantbenefit. org/

European calculators • https: //www. efclif. com/scientific-activity/score-calculators/clif-c -aclf • https: //www. efclif. com/scientific-activity/score-calculators/clif-c -ad

What is the future of liver allocation? • A different way of modelling (artificial intelligence) • Consider the use of expanded criteria grafts (DCD, Normothermic regional perfusion and ex-situ liver perfusion) • Allow for expansion of recipient selection criteria (colorectal metastasis, HCC beyond Milan criteria, cholangiocarcinoma) • Interaction with living donor transplantation • Going beyond traditional outcomes – quality of life measures

 Farzad samie
Farzad samie Farzad khorasani
Farzad khorasani Linked allocation
Linked allocation Bone marrow transplantation sri lanka
Bone marrow transplantation sri lanka Law of transplantation
Law of transplantation Patrick evrard mont godinne
Patrick evrard mont godinne Cultural transposition
Cultural transposition How does a kidney transplant work
How does a kidney transplant work Stem cell or bone marrow transplantation bangkok
Stem cell or bone marrow transplantation bangkok Decision support systems and intelligent systems
Decision support systems and intelligent systems Dicapine
Dicapine Embedded systems vs cyber physical systems
Embedded systems vs cyber physical systems Engineering elegant systems: theory of systems engineering
Engineering elegant systems: theory of systems engineering Decompensated liver disease
Decompensated liver disease Heterogeneous hypoechoic lesion in liver
Heterogeneous hypoechoic lesion in liver Vascolarizzazione pancreas
Vascolarizzazione pancreas Where is bile secreted
Where is bile secreted