8 11 NOTES Attractive Intermolecular Forces Attractive Intermolecular


- Slides: 10

8. 11 – NOTES Attractive (Intermolecular) Forces

• Attractive (Intermolecular) Forces: forces that exist between molecules that contribute to the properties of the substance. • Intramolecular vs. Intermolecular • Intramolecular Forces: Forces of Attraction within the molecule. • Examples: covalent, ionic, and metallic bonds. • Intermolecular Forces: Forces of Attraction in between molecules • Also called Van der Waals Forces. • Examples: London Dispersion, Dipole-Dipole, and Hydrogen Bonding. • Intramolecular Forces are stronger than Intermolecular Forces.

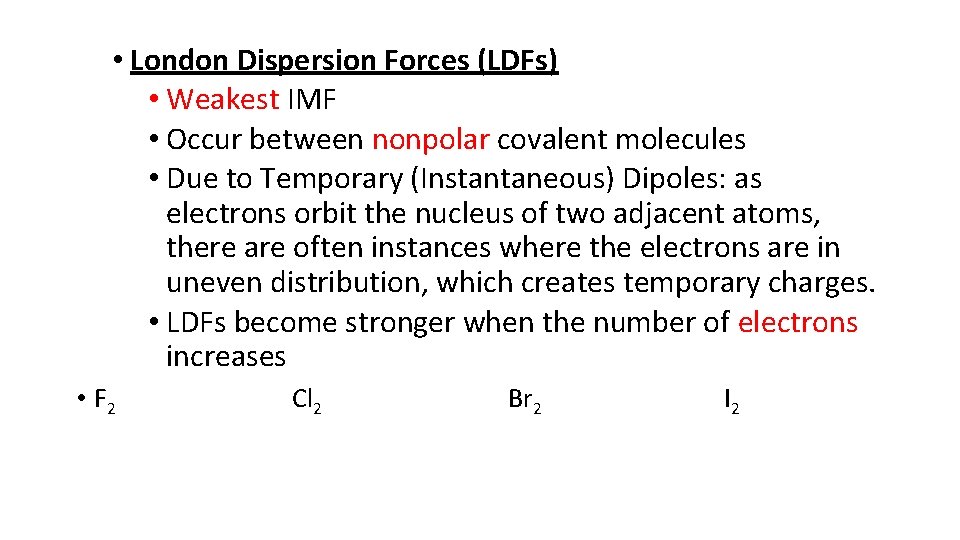
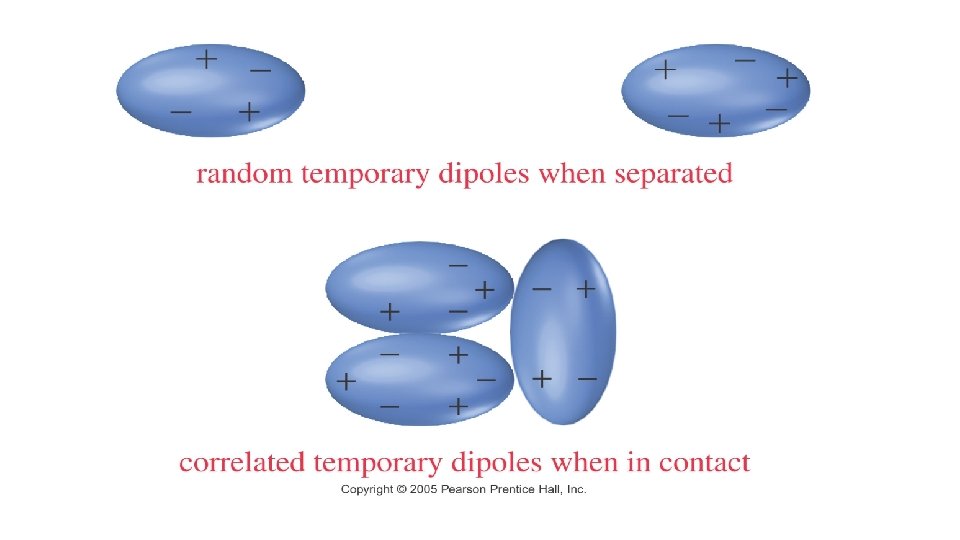
• London Dispersion Forces (LDFs) • Weakest IMF • Occur between nonpolar covalent molecules • Due to Temporary (Instantaneous) Dipoles: as electrons orbit the nucleus of two adjacent atoms, there are often instances where the electrons are in uneven distribution, which creates temporary charges. • LDFs become stronger when the number of electrons increases • F 2 Cl 2 Br 2 I 2


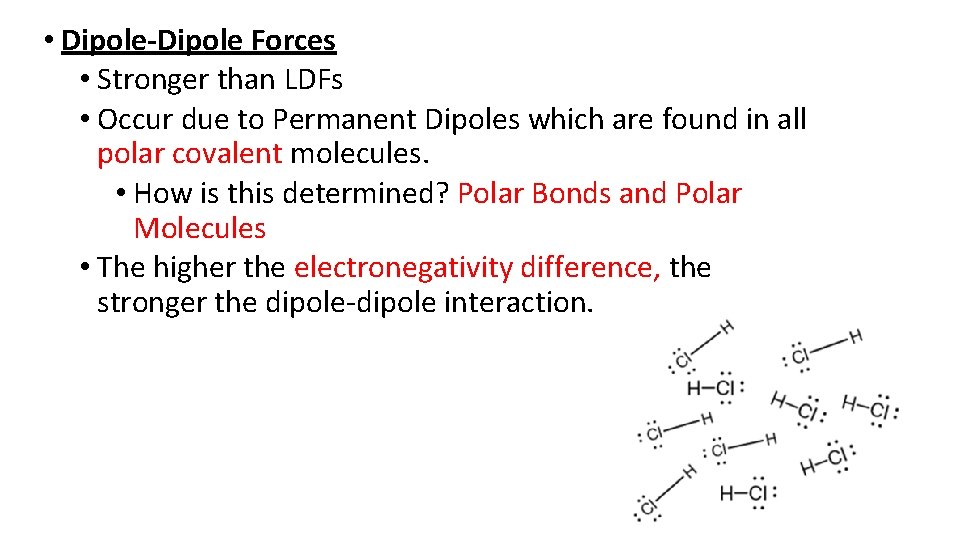
• Dipole-Dipole Forces • Stronger than LDFs • Occur due to Permanent Dipoles which are found in all polar covalent molecules. • How is this determined? Polar Bonds and Polar Molecules • The higher the electronegativity difference, the stronger the dipole-dipole interaction.

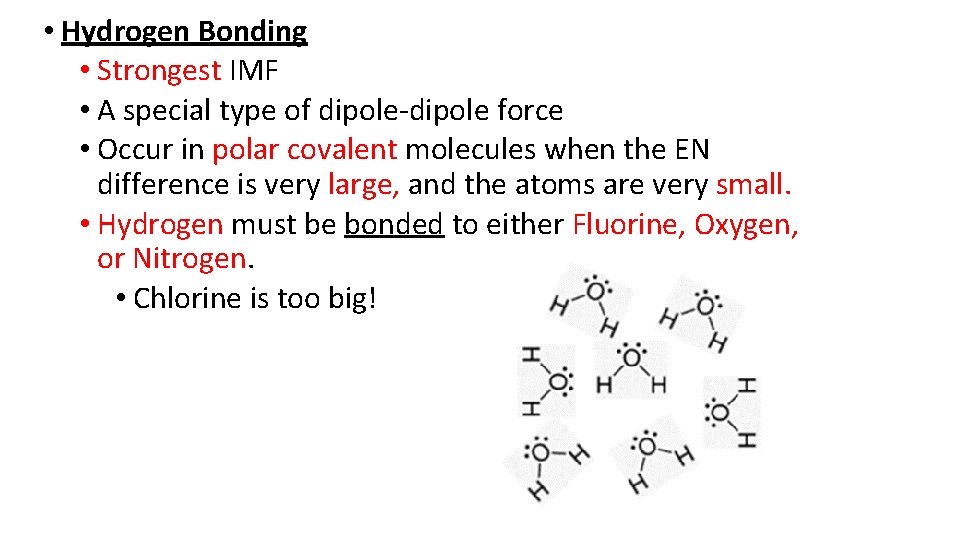
• Hydrogen Bonding • Strongest IMF • A special type of dipole-dipole force • Occur in polar covalent molecules when the EN difference is very large, and the atoms are very small. • Hydrogen must be bonded to either Fluorine, Oxygen, or Nitrogen. • Chlorine is too big!

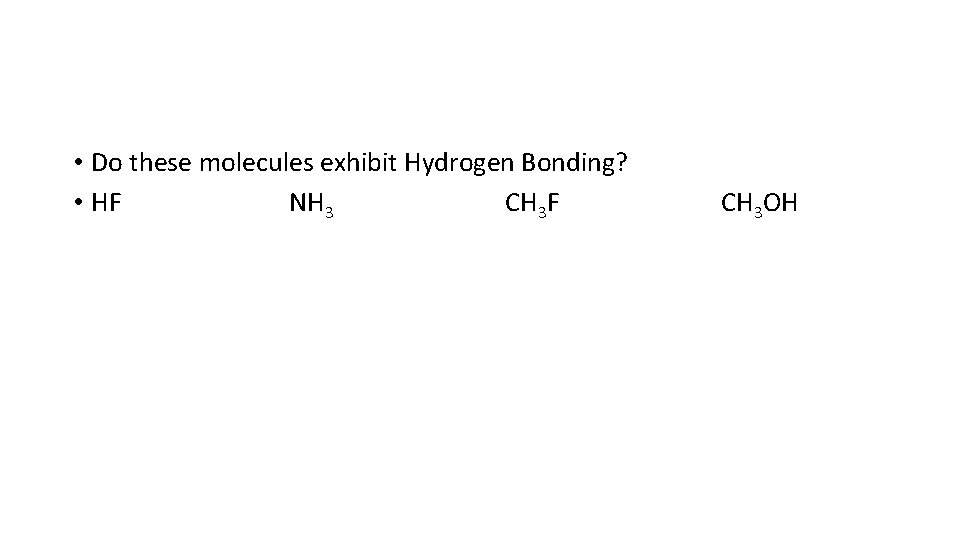
• Do these molecules exhibit Hydrogen Bonding? • HF NH 3 CH 3 F CH 3 OH

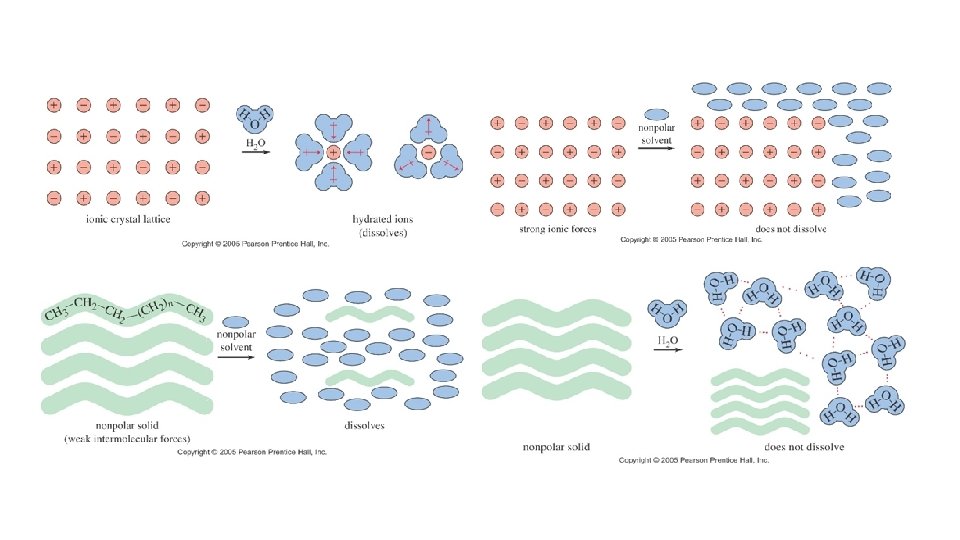
• Properties of Covalent (Molecular) Substances • Molecular Substances vary in state at room temperature • Many are solid: sugars, rubber, wax, I 2 • Some are liquid: water, alcohols, some fuels (octane), Br 2 • Most are gases: CO 2, SO 2, CO, Cl 2, F 2 • Regardless of state, molecular substances have low MPs and BPs. • Most are insoluble in water • Polar Solutes can dissolve in polar solvents (H 2 O) • Nonpolar Solutes can dissolve in nonpolar solvents (Hexane, CCl 4, etc. ) • Most are non-conductors and relatively soft • Some polar molecules conduct when dissolved in water (i. e. acids and bases)


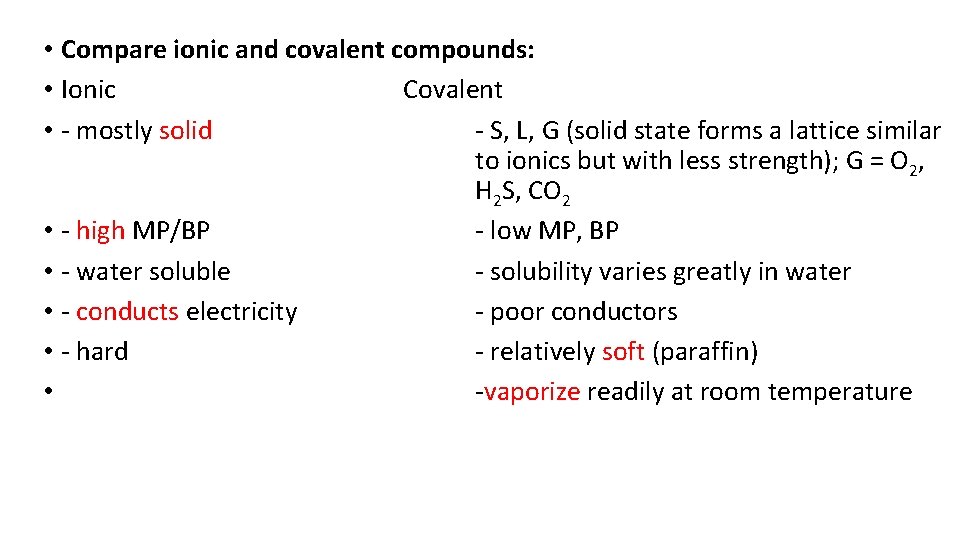
• Compare ionic and covalent compounds: • Ionic Covalent • - mostly solid - S, L, G (solid state forms a lattice similar to ionics but with less strength); G = O 2, H 2 S, CO 2 • - high MP/BP - low MP, BP • - water soluble - solubility varies greatly in water • - conducts electricity - poor conductors • - hard - relatively soft (paraffin) • -vaporize readily at room temperature