64 th International Symposium on Molecular Spectroscopy June







- Slides: 14

64 th International Symposium on Molecular Spectroscopy June 26, 2009 The Pure Rotational Spectrum of Zn. S (X 1 +) Lindsay N. Zack Lucy M. Ziurys Department of Chemistry, Department of Astronomy, Steward Observatory, and Arizona Radio Observatory University of Arizona

64 th International Symposium on Molecular Spectroscopy June 26, 2009 Previous Work • Mass spectrometry – Dissociation energy (Marquart and Berkowitz 1963; de Maria et al. 1965) • Spectroscopic measurements – Absorption spectroscopy of Zn. S in l 7000 -1900 region (Sen-Gupta 1933) – X-ray emission and luminescence spectroscopy of crystals and nanoparticles (Laihia et al. 1996; Denzler et al. 1998) • Theoretical calculations – Different levels of theory with and without relativistic corrections (e. g. Bauschlicher and Langhoff 1986; Peterson et al. 2007) – Similarities between single molecule and bulk properties (Anderson et al. 1987; Bridgeman and Rothery 2000; Chambaud et al. 2008) – re ~ 2. 04 – 2. 12 Å

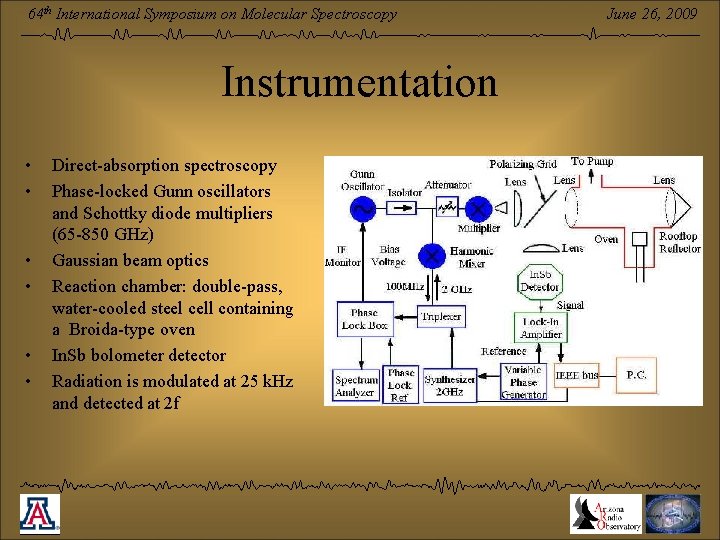
64 th International Symposium on Molecular Spectroscopy Instrumentation • • • Direct-absorption spectroscopy Phase-locked Gunn oscillators and Schottky diode multipliers (65 -850 GHz) Gaussian beam optics Reaction chamber: double-pass, water-cooled steel cell containing a Broida-type oven In. Sb bolometer detector Radiation is modulated at 25 k. Hz and detected at 2 f June 26, 2009

64 th International Symposium on Molecular Spectroscopy Direct-absorption mm/sub-mm wave spectrometer June 26, 2009

64 th International Symposium on Molecular Spectroscopy Molecular Synthesis • Gas-phase synthesis • Zinc vapor produced in Broida-type oven – Alumina crucible in tungsten wire basket – m. p. 420 C • H 2 S added over top of oven • Argon carrier gas • d. c. discharge needed (250 m. A at 200 V) June 26, 2009

64 th International Symposium on Molecular Spectroscopy June 26, 2009 42 GHz (~7 B) initially scanned continuosly

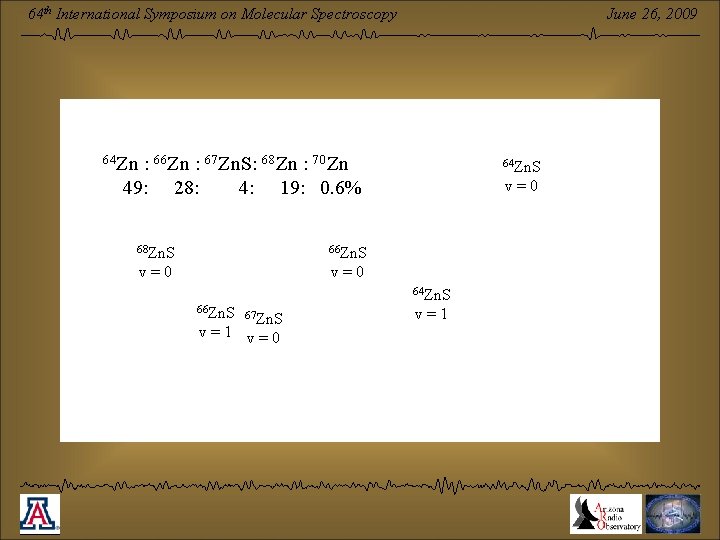
June 26, 2009 64 th International Symposium on Molecular Spectroscopy 64 Zn : 66 Zn : 67 Zn. S: 68 Zn : 70 Zn 49: 28: 4: 19: 0. 6% 68 Zn. S 66 Zn. S v=0 64 Zn. S 66 Zn. S 67 Zn. S v=1 v=0 v=1

64 th International Symposium on Molecular Spectroscopy June 26, 2009

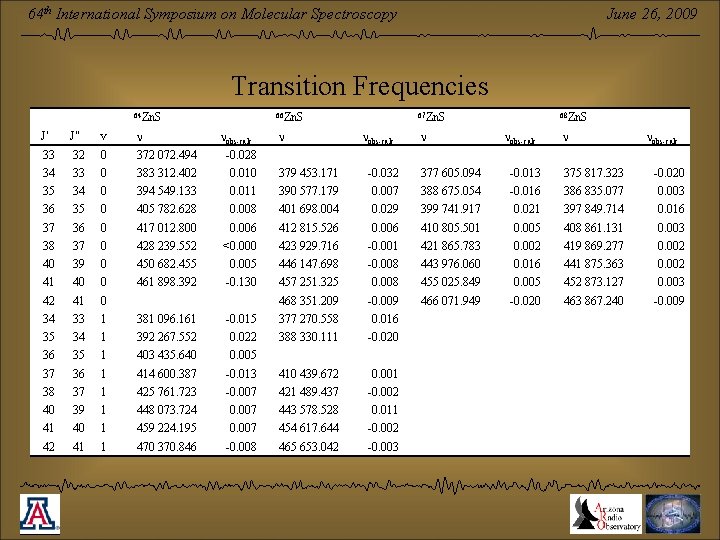
June 26, 2009 64 th International Symposium on Molecular Spectroscopy Transition Frequencies 64 Zn. S 66 Zn. S 0 nobs-calc -0. 028 33 0 383 312. 402 0. 010 379 453. 171 -0. 032 377 605. 094 -0. 013 375 817. 323 -0. 020 35 34 0 394 549. 133 0. 011 390 577. 179 0. 007 388 675. 054 -0. 016 386 835. 077 0. 003 36 35 0 405 782. 628 0. 008 401 698. 004 0. 029 399 741. 917 0. 021 397 849. 714 0. 016 37 36 0 417 012. 800 0. 006 412 815. 526 0. 006 410 805. 501 0. 005 408 861. 131 0. 003 38 37 0 428 239. 552 <0. 000 423 929. 716 -0. 001 421 865. 783 0. 002 419 869. 277 0. 002 40 39 0 450 682. 455 0. 005 446 147. 698 -0. 008 443 976. 060 0. 016 441 875. 363 0. 002 41 40 0 461 898. 392 -0. 130 457 251. 325 0. 008 455 025. 849 0. 005 452 873. 127 0. 003 42 41 0 468 351. 209 -0. 009 466 071. 949 -0. 020 463 867. 240 -0. 009 34 33 1 381 096. 161 -0. 015 377 270. 558 0. 016 35 34 1 392 267. 552 0. 022 388 330. 111 -0. 020 36 35 1 403 435. 640 0. 005 37 36 1 414 600. 387 -0. 013 410 439. 672 0. 001 38 37 1 425 761. 723 -0. 007 421 489. 437 -0. 002 40 39 1 448 073. 724 0. 007 443 578. 528 0. 011 41 40 1 459 224. 195 0. 007 454 617. 644 -0. 002 42 41 1 470 370. 846 -0. 008 465 653. 042 -0. 003 J” v 33 32 34 nobs-calc n 68 Zn. S n 372 072. 494 J’ n 67 Zn. S nobs-calc n nobs-calc

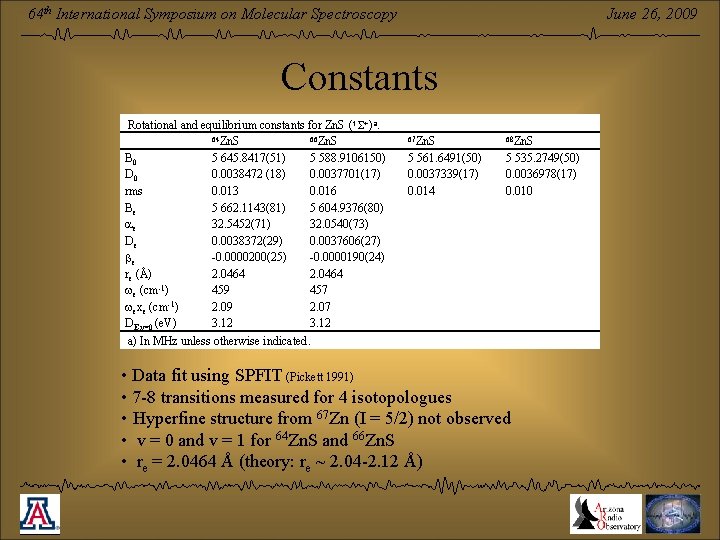
June 26, 2009 64 th International Symposium on Molecular Spectroscopy Constants Rotational and equilibrium constants for Zn. S (1 +)a. 64 Zn. S 66 Zn. S B 0 5 645. 8417(51) 5 588. 9106150) D 0 0. 0038472 (18) 0. 0037701(17) rms 0. 013 0. 016 Be 5 662. 1143(81) 5 604. 9376(80) 32. 5452(71) 32. 0540(73) ae De 0. 0038372(29) 0. 0037606(27) -0. 0000200(25) -0. 0000190(24) be re (Å) 2. 0464 -1 we (cm ) 459 457 -1 wexe (cm ) 2. 09 2. 07 DE, v=0 (e. V) 3. 12 a) In MHz unless otherwise indicated. 67 Zn. S 68 Zn. S 5 561. 6491(50) 0. 0037339(17) 0. 014 5 535. 2749(50) 0. 0036978(17) 0. 010 • Data fit using SPFIT (Pickett 1991) • 7 -8 transitions measured for 4 isotopologues • Hyperfine structure from 67 Zn (I = 5/2) not observed • v = 0 and v = 1 for 64 Zn. S and 66 Zn. S • re = 2. 0464 Å (theory: re ~ 2. 04 -2. 12 Å)

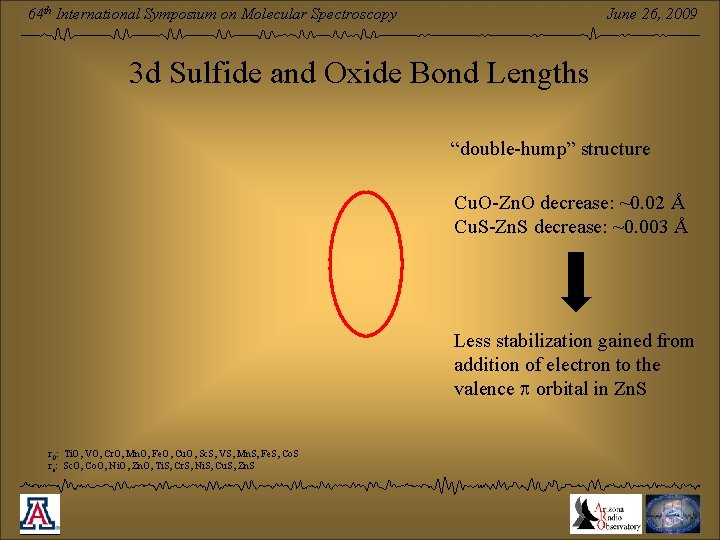
June 26, 2009 64 th International Symposium on Molecular Spectroscopy 3 d Sulfide and Oxide Bond Lengths “double-hump” structure Cu. O-Zn. O decrease: ~0. 02 Å Cu. S-Zn. S decrease: ~0. 003 Å Less stabilization gained from addition of electron to the valence p orbital in Zn. S r 0: Ti. O, VO, Cr. O, Mn. O, Fe. O, Cu. O, Sc. S, VS, Mn. S, Fe. S, Co. S re: Sc. O, Co. O, Ni. O, Zn. O, Ti. S, Cr. S, Ni. S, Cu. S, Zn. S

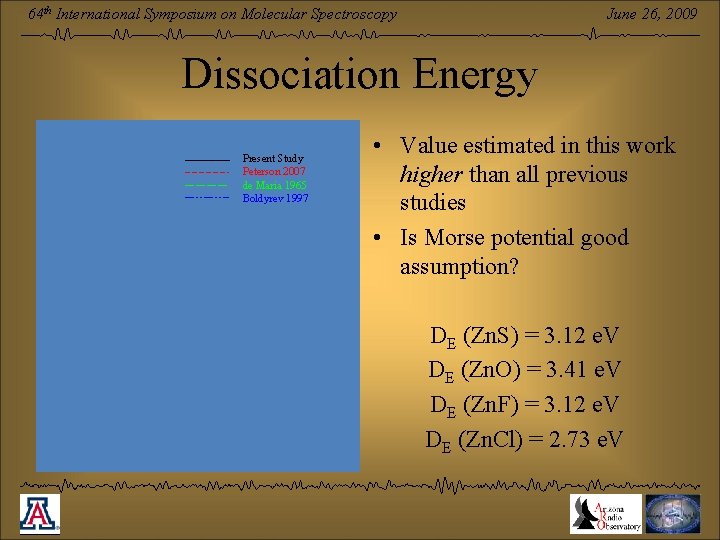
June 26, 2009 64 th International Symposium on Molecular Spectroscopy Dissociation Energy Present Study Peterson 2007 de Maria 1965 Boldyrev 1997 • Value estimated in this work higher than all previous studies • Is Morse potential good assumption? DE (Zn. S) = 3. 12 e. V DE (Zn. O) = 3. 41 e. V DE (Zn. F) = 3. 12 e. V DE (Zn. Cl) = 2. 73 e. V

64 th International Symposium on Molecular Spectroscopy June 26, 2009 Summary • Rotational spectra of four isotopologues of Zn. S have been measured • Rotational and equilibrium constants have been determined • This work agrees well with theory with regards to bond lengths • Dissociation energy is significantly higher than previous theoretical and experimental (mass spec) studies indicate • Similarities in bonding trends between oxides and sulfides

64 th International Symposium on Molecular Spectroscopy June 26, 2009 Acknowledgements • Professor Lucy Ziurys • Dr. De. Wayne Halfen • Robin Pulliam, Brent Harris, Ming Sun, Emmy Tenenbaum, Jessica Dodd, Gilles Adande, Matthew Bucchino, and Jie Min • Funding- NSF and NASA
 International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Catalysis lecture notes
Catalysis lecture notes Upcdms
Upcdms Applications of uv and visible spectroscopy
Applications of uv and visible spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International police executive symposium
International police executive symposium Ips perforating
Ips perforating Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure