2020 SCAI Guidelines on Device Selection in AortoIliac






- Slides: 21

2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions Dmitriy N. Feldman, MD, FACC, FSCAI, FSVM Director, Endovascular Service Director, Interventional Observation/Telemetry Unit Associate Professor of Medicine Interventional Cardiac and Endovascular Laboratory Weill Cornell Medical College, New York Presbyterian Hospital May 14 th, 2020

Disclosures No relevant disclosures

GUIDELINES: Symptomatic Patients 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Arterial Disease

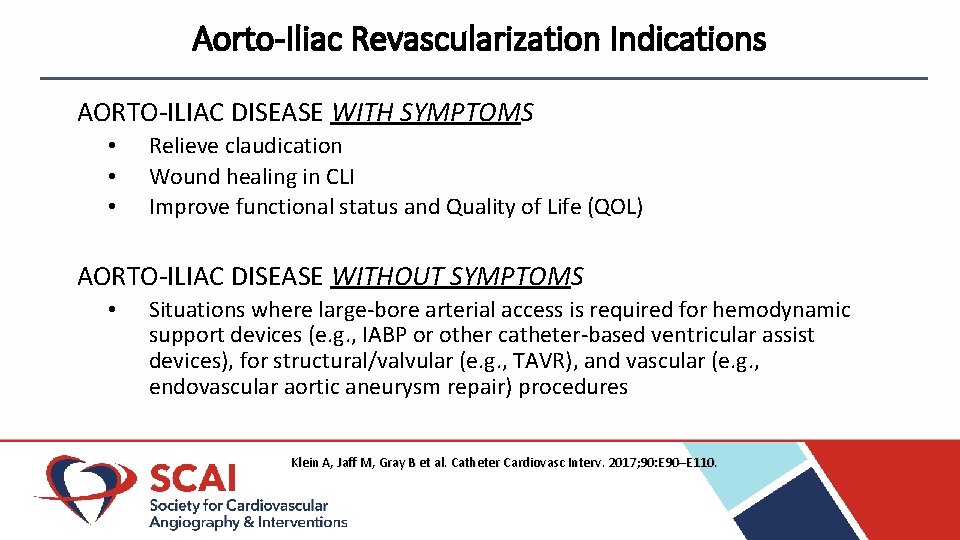
Aorto-Iliac Revascularization Indications AORTO-ILIAC DISEASE WITH SYMPTOMS • • • Relieve claudication Wound healing in CLI Improve functional status and Quality of Life (QOL) AORTO-ILIAC DISEASE WITHOUT SYMPTOMS • Situations where large-bore arterial access is required for hemodynamic support devices (e. g. , IABP or other catheter-based ventricular assist devices), for structural/valvular (e. g. , TAVR), and vascular (e. g. , endovascular aortic aneurysm repair) procedures Klein A, Jaff M, Gray B et al. Catheter Cardiovasc Interv. 2017; 90: E 90–E 110.

Rise of endovascular interventions – patient preference

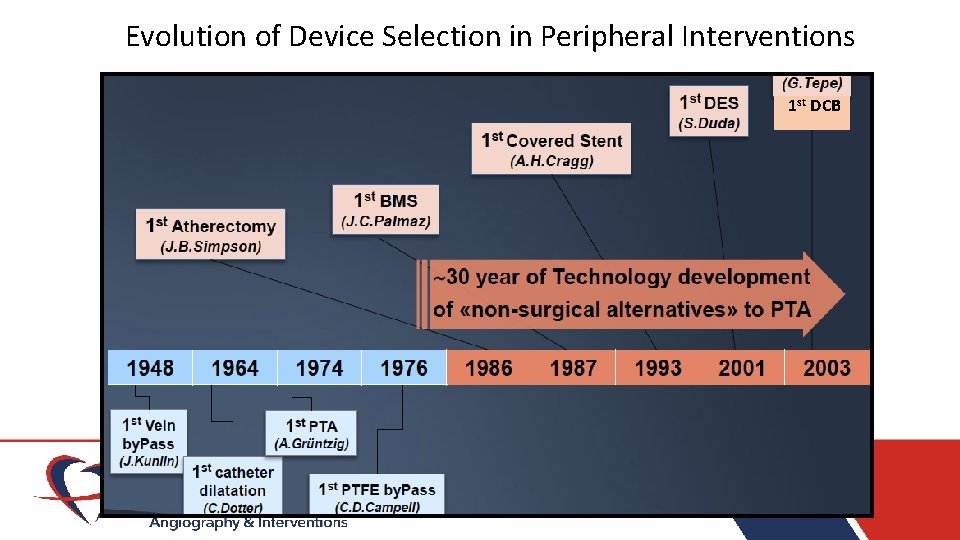
Evolution of Device Selection in Peripheral Interventions 1 st DCB

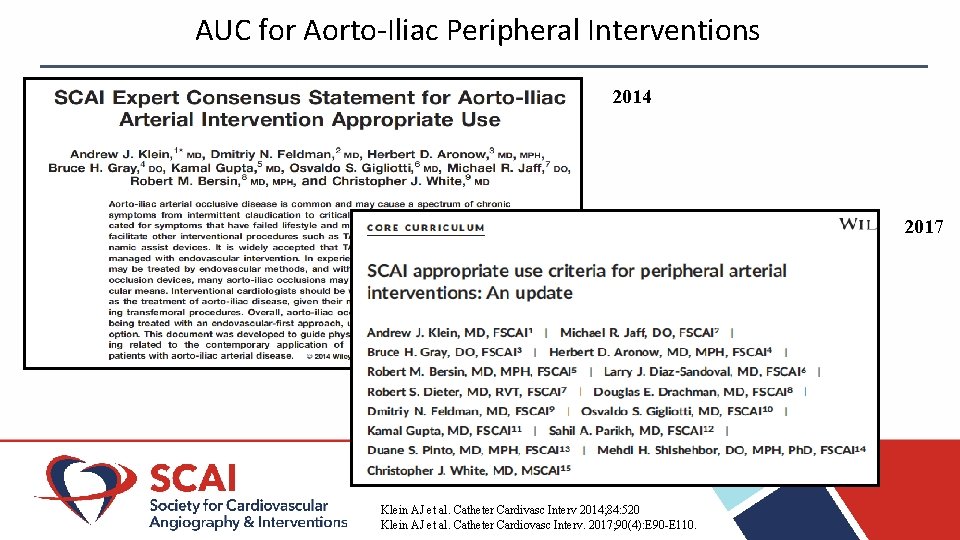
AUC for Aorto-Iliac Peripheral Interventions 2014 2017 Klein AJ et al. Catheter Cardivasc Interv 2014; 84: 520 Klein AJ et al. Catheter Cardiovasc Interv. 2017; 90(4): E 90 -E 110.

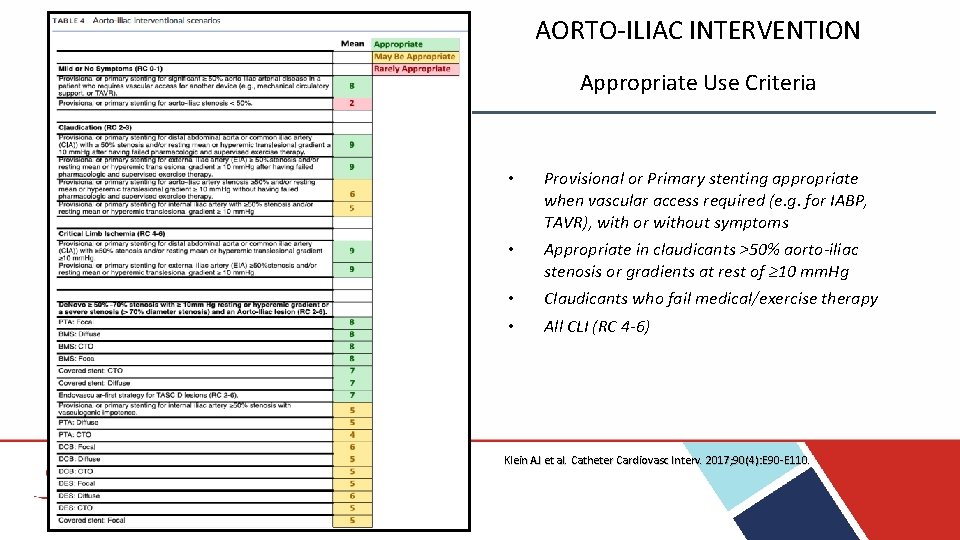
AORTO-ILIAC INTERVENTION Appropriate Use Criteria • Provisional or Primary stenting appropriate when vascular access required (e. g. for IABP, TAVR), with or without symptoms • Appropriate in claudicants >50% aorto-iliac stenosis or gradients at rest of ≥ 10 mm. Hg • • Claudicants who fail medical/exercise therapy All CLI (RC 4 -6) Klein AJ et al. Catheter Cardiovasc Interv. 2017; 90(4): E 90 -E 110.

Bailey SR et al. J Am Coll Cardiol. 2019 Jan 22; 73(2): 214 -237

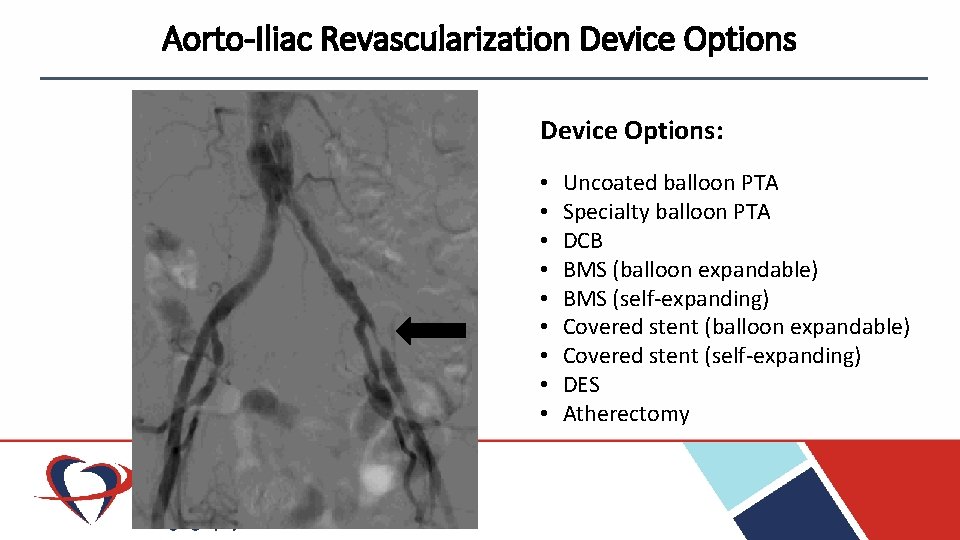
Aorto-Iliac Revascularization Device Options: • • • Uncoated balloon PTA Specialty balloon PTA DCB BMS (balloon expandable) BMS (self-expanding) Covered stent (balloon expandable) Covered stent (self-expanding) DES Atherectomy

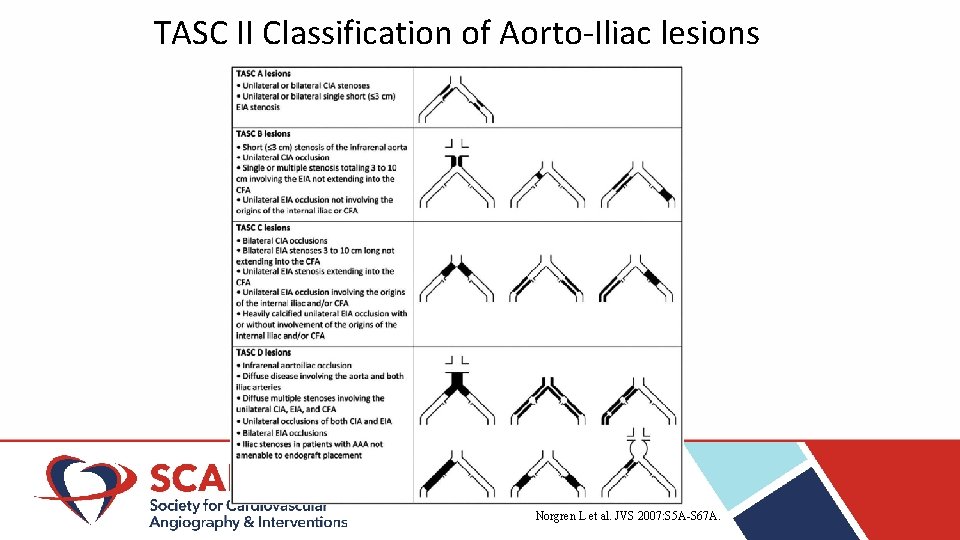
TASC II Classification of Aorto-Iliac lesions Norgren L et al. JVS 2007: S 5 A-S 67 A.

2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions • Provide a comprehensive review of comparative effectiveness data in aorto-iliac arterial interventions • Focus on safety and efficacy of groups of devices • The cost of the devices is considered secondary to examining efficacy and safety data • Provide clinicians with guidance (class of recommendation and level of evidence) for device selection, when these devices are intended as definitive therapy.

2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions Feldman DN et al. Catheter Cardiovasc Interv 2020 Study Selection Process

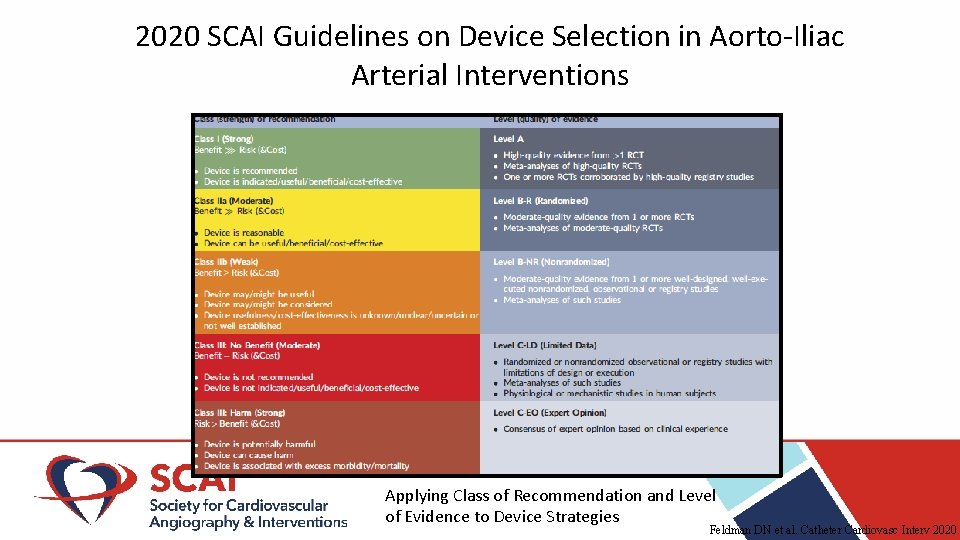
2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions Applying Class of Recommendation and Level of Evidence to Device Strategies Feldman DN et al. Catheter Cardiovasc Interv 2020

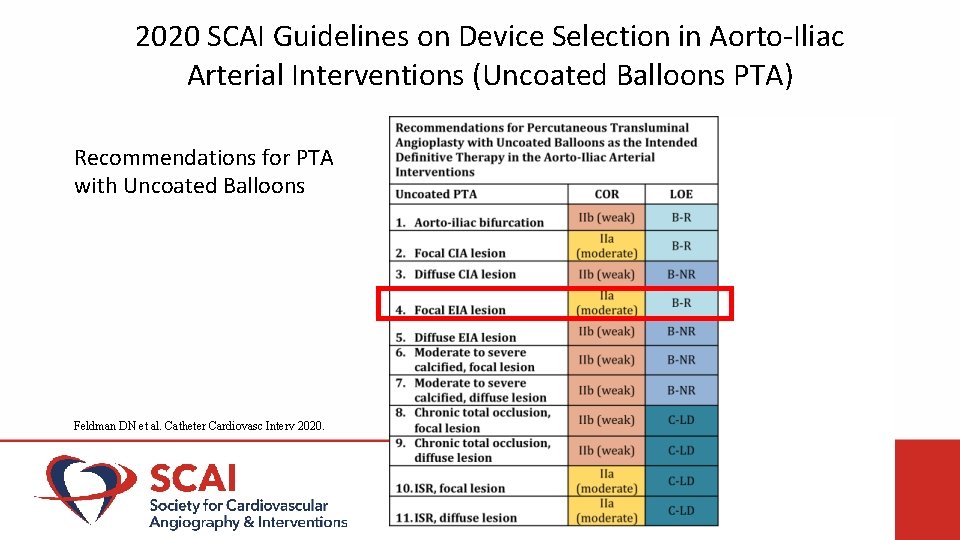
2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions (Uncoated Balloons PTA) Recommendations for PTA with Uncoated Balloons Feldman DN et al. Catheter Cardiovasc Interv 2020.

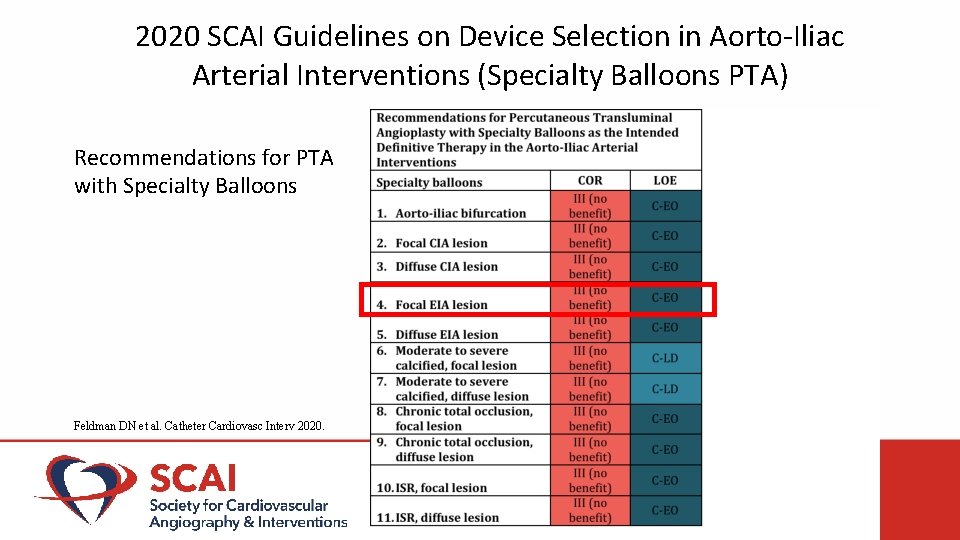
2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions (Specialty Balloons PTA) Recommendations for PTA with Specialty Balloons Feldman DN et al. Catheter Cardiovasc Interv 2020.

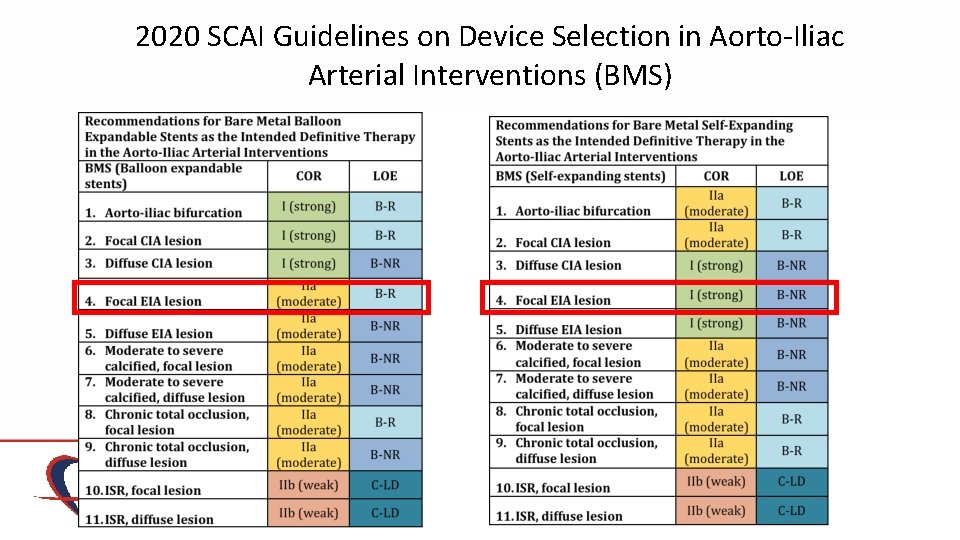
2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions (BMS)

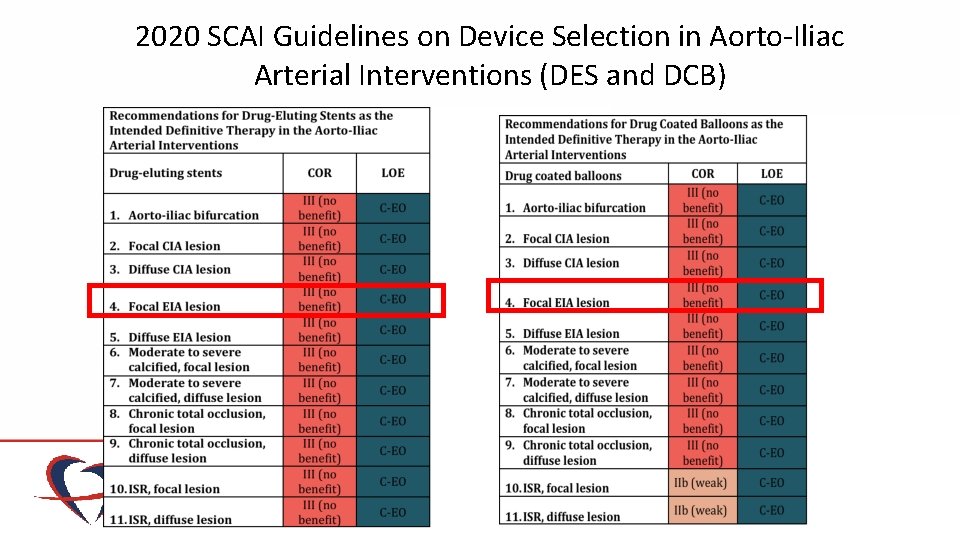
2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions (DES and DCB)

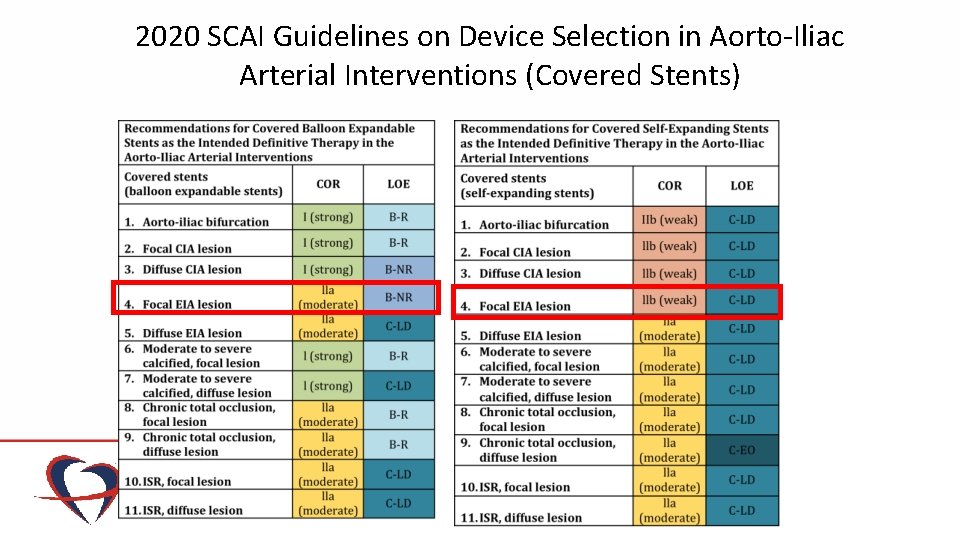
2020 SCAI Guidelines on Device Selection in Aorto-Iliac Arterial Interventions (Covered Stents)

Conclusions • PVI is a very effective therapy for patients with lifestyle-limiting claudication and hemodynamically significant Aorto-Iliac disease. • The role of PVI is symptomatic and functional status improvement. • SCAI 2017 AUC and 2018 ACC/AHA/SCAI/SIR/SVM AUC were developed to provide clinicians with guidance regarding appropriateness of PVI for specific clinical and anatomical criteria. • 2020 SCAI guidelines document provides comprehensive guidance with specific recommendations (COR and LOE) for Aorto-Iliac device selection, when these devices are intended as the definitive therapy.

Thank you!