2005 Full Year Results Frank Condella Chief Executive
































- Slides: 55

2005 Full Year Results Frank Condella Chief Executive Officer Donald Nicholson Finance Director 19 April 2006 NASDAQ: SKYE LSE: SKP www. skyepharma. com UK tel: +44 (0)207 491 1777 US tel: +1 (212) 753 5780

Legal statement This presentation does not constitute or form part of any offer for sale or subscription or solicitation of any offer to buy or subscribe for any securities in Skye. Pharma PLC nor shall it or any part of it form the basis of or be relied on in connection with any contract or commitment whatsoever. This presentation is being made only to and is directed at (a) persons who have professional experience in matters relating to investments who fall within Article 19(1) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”); or (b) high net worth entities and other persons to whom it may otherwise lawfully be communicated, falling within Article 49(1) of the Order (all such persons together being referred to as “relevant persons”). Any person who is not a relevant person should not rely on this presentation or any of its contents. This presentation includes certain forward-looking statements with respect to certain development projects, potential collaborative partnerships, results of operations and certain plans and objectives of Skye. Pharma including, in particular, the statements regarding potential sales revenues from Paxil CR, targeted sales revenues from other products both currently marketed and under development, possible launch dates for new products, and our revenue and profit guidance for the 2005 financial year. By their very nature forward-looking statements involve risk and uncertainty that could cause actual results and developments to differ materially from those expressed or implied. The significant risks related to Skye. Pharma’s business which could cause our actual results and developments to differ materially from those forward-looking statements are discussed in Skye. Pharma’s SEC filings under the caption “Risk Factors”. Skye. Pharma reports under IFRS. Where US dollar equivalents have been provided for convenience in this presentation, a fixed exchange rate of $1. 77 = £ 1 has been used throughout. These dollar equivalent numbers do not imply restatement from IFRS to US GAAP. This presentation was updated on 18 Apr 2006 2

Frank Condella Chief Executive Officer 3

Headline news 2005 Ø difficult year for Skye. Pharma Ø did not conclude development agreement for Flutiform™ Ø merger discussions absorbed a lot of time and resource Ø rights issue to raise funds primarily for Flutiform Ph III trials Ø Ph III started on time Feb ‘ 06 Ø approach by Innovata PLC Ø company entered strategic review period 4

Headline news 2005 Ø Triglide launched in US Ø new agreement with Glaxo. Smith. Kline on Paxil CR Ø advanced UK marketing authorisation application for Depo. Dur Ø Depo. Bupivacaine licensed to Mundipharma and Maruho Ø all territories outside North America 5

2005 results summary Ø revenues £ 61 m (2004: £ 75 m) down by £ 14 m (-18%) Ø milestone income affected by timing of key deals & product approvals Ø Paxil CR royalties hit by supply problems Ø excl Paxil CR, royalty income up by 38% Ø op loss pre-exceps £ 16 m (2004 loss: £ 0. 4 m) Ø £ 21 m exceptional write-off Ø net loss £ 51 m (2004 loss: £ 19 m) Ø net loss per share 8 p (2004 loss: 3 p) Ø end-2005 cash £ 34 m (end-2004 £ 15 m) Ø £ 54 m raised through rights and convertible issues 6

Headline news 2006 YTD Ø strategic review completed Ø founder Ian Gowrie-Smith resigned from the Board Ø Jerry Karabelas appointed non-executive Chairman Ø new management team appointed Ø strategic plan announced Ø EGM completed and supported new direction 7

Strategic plan 2006 Ø new leadership Ø divest injectables unit Ø strengthen balance sheet Ø reduce cash burn Ø continue phase III for Flutiform™ and outlicense this year Ø focus on core oral/inhalation unit and expand pipeline Ø improve operational efficiency Ø longer term aim to market own products in selected therapeutic area 8

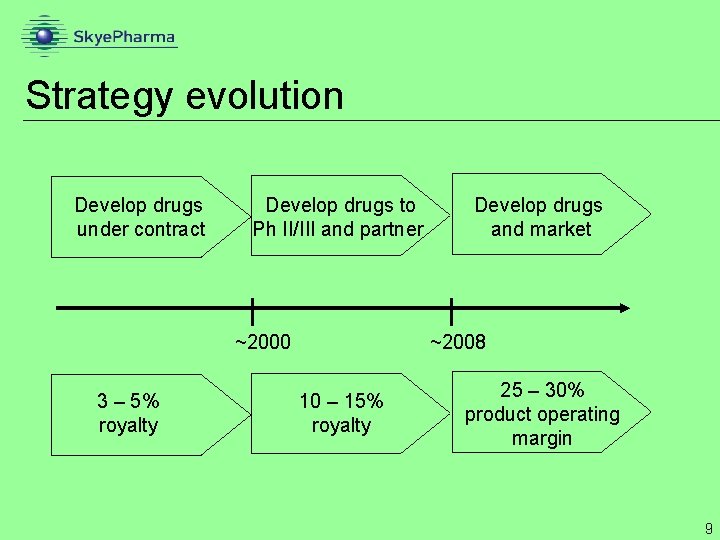
Strategy evolution Develop drugs under contract Develop drugs to Ph II/III and partner ~2000 3 – 5% royalty Develop drugs and market ~2008 10 – 15% royalty 25 – 30% product operating margin 9

New management team Jerry Karabelas Non-exec Chairman Novartis; Smith. Kline Beecham Frank Condella Chief Executive IVAX; Faulding; Roche Ken Cunningham Chief Operating Officer Arakis; Alza; Sequus Donald Nicholson Finance Director Boehringer-Mannheim; Wellcome John Murphy General Counsel Celltech; Medeva Francesco Patalano President, Europe Novartis; CIBA; Fisons 10

Sale of injectables unit - update Ø due diligence materials prepared Ø UBS selected as investment bank Ø several parties actively engaged in process Ø management presentations and due diligence ongoing 11

Core Oral / Inhalation Business 12

Major marketed products Oral/inhalation: Paxil CR (Glaxo. Smith. Kline) Triglide (First Horizon) Xatral OD / Uroxatral (Sanofi-Aventis) Solaraze (Bradley; Shire) 13

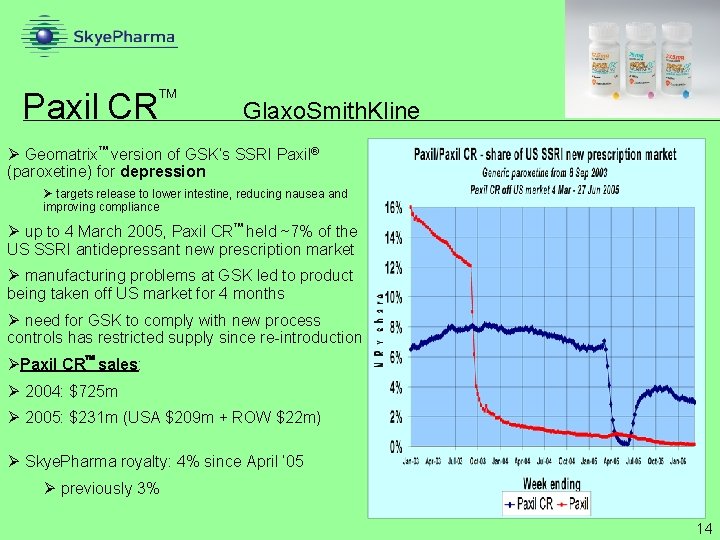
Paxil CR Glaxo. Smith. Kline Ø Geomatrix version of GSK’s SSRI Paxil® (paroxetine) for depression Ø targets release to lower intestine, reducing nausea and improving compliance Ø up to 4 March 2005, Paxil CR held ~7% of the US SSRI antidepressant new prescription market Ø manufacturing problems at GSK led to product being taken off US market for 4 months Ø need for GSK to comply with new process controls has restricted supply since re-introduction ØPaxil CR sales: Ø 2004: $725 m Ø 2005: $231 m (USA $209 m + ROW $22 m) Ø Skye. Pharma royalty: 4% since April ’ 05 Ø previously 3% 14

Paxil CR – generic competition Ø Mylan has filed a Para IV certification for “paroxetine hydrochloride controlled release” Ø GSK’s hydrochloride hemihydrate patent (expires June ’ 07) not challenged Ø GSK has decided NOT to sue for patent infringement against patents listed in FDA Orange Book Ø no 30 month stay of FDA approval Ø Skye. Pharma has several patents on technology in Paxil CR™ not listed in FDA Orange Book Ø our policy is to enforce patents wherever possible 15

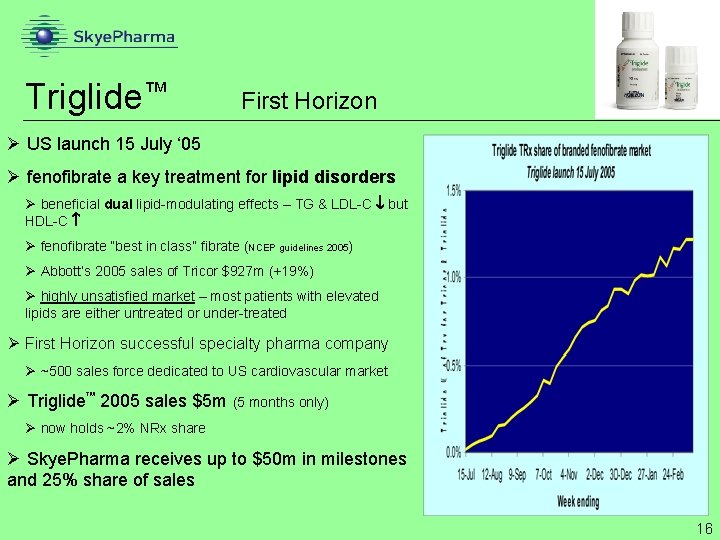
Triglide™ First Horizon Ø US launch 15 July ‘ 05 Ø fenofibrate a key treatment for lipid disorders Ø beneficial dual lipid-modulating effects – TG & LDL-C but HDL-C Ø fenofibrate “best in class” fibrate (NCEP guidelines 2005) Ø Abbott’s 2005 sales of Tricor $927 m (+19%) Ø highly unsatisfied market – most patients with elevated lipids are either untreated or under-treated Ø First Horizon successful specialty pharma company Ø ~500 sales force dedicated to US cardiovascular market Ø Triglide 2005 sales $5 m (5 months only) Ø now holds ~2% NRx share Ø Skye. Pharma receives up to $50 m in milestones and 25% share of sales 16

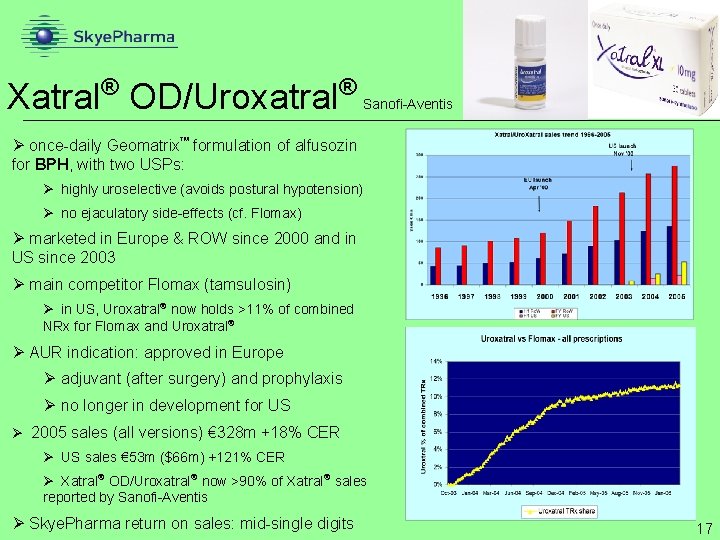
® Xatral OD/Uroxatral ® Sanofi-Aventis Ø once-daily Geomatrix formulation of alfusozin for BPH, with two USPs: Ø highly uroselective (avoids postural hypotension) Ø no ejaculatory side-effects (cf. Flomax) Ø marketed in Europe & ROW since 2000 and in US since 2003 Ø main competitor Flomax (tamsulosin) Ø in US, Uroxatral now holds >11% of combined NRx for Flomax and Uroxatral Ø AUR indication: approved in Europe Ø adjuvant (after surgery) and prophylaxis Ø no longer in development for US Ø 2005 sales (all versions) € 328 m +18% CER Ø US sales € 53 m ($66 m) +121% CER Ø Xatral OD/Uroxatral now >90% of Xatral sales reported by Sanofi-Aventis Ø Skye. Pharma return on sales: mid-single digits 17

Solaraze Bradley / Shire Ø topical gel formulation of diclofenac for actinic keratosis Ø AK is early form of squamous cell carcinoma Ø alternative treatments for AK are painful and disfiguring Ø Skye. Pharma formulation retains high concentration of active in upper skin layers Ø marketed by Bradley in North America, Shire in Europe / Australasia Ø 2005 global in-market sales $27. 5 mn (+78%) Ø US $10 m in 9 M ‘ 05 (latest data reported by Bradley) – FY ‘ 05 est $15 m Ø vs $6 m in 2004 (for 4 months) Ø Europe/Ro. W $12. 5 m (+32%) Ø Australian marketing authorisation process has advanced Ø Skye. Pharma royalty: mid-teens 18

Key near-term pipeline products Oral/inhalation: Foradil Certihaler Pulmicort HFA Requip Once-a-day Flutiform 19

Foradil Certihaler ™ Novartis / Schering-Plough Ø long-acting bronchodilator formoterol in multi-dose dry-powder inhaler Ø Skye. Pharma developed both device and formulation Ø also used in second collaboration with Novartis (QAB 149, indacaterol) Ø Schering-Plough to market in key US market, Novartis elsewhere Ø 2005 sales of Foradil in Aeroliser single-dose DPI: $332 m Ø Foradil Certihaler™ now approved in 22 markets (Europe, Mid-East, S America…) Ø launched in Germany Sep ’ 05 and Switzerland Oct ’ 05 but recalled from both markets Jan ’ 06 Ø a few patients experienced accidental incorrect dose Ø Skye. Pharma working with Novartis to investigate cause and correct Ø FDA “approvable” letter Apr ’ 06 – device modifications will be required for final approval Ø Skye. Pharma return on sales: ~10% (royalty + manufacturing return) 20

Pulmicort® HFA-MDI Astra. Zeneca (for Europe) Ø Pulmicort® (budesonide) in CFC-free MDI Ø inhaled steroid for asthma Ø will allow AZN to withdraw CFC-MDI version (Montreal protocol requirement) Ø Skye. Pharma developed formulation and conducted clinical development for AZN Ø filed on country-by-country basis in Europe mid ’ 05 Ø first European approval (Finland) Feb ’ 06 Ø double digit royalty 21

Requip Once-a-day Glaxo. Smith. Kline Ø once-daily oral dosage formulation of ropinirole (dopamine agonist) for Parkinson’s disease Ø NB another controlled release formulation of ropinirole in development by GSK for restless leg syndrome does NOT use Skye. Pharma technology Ø dopamine agonists increasingly recommended as first-line therapy Ø once-daily version should deliver efficacy and provide convenience Ø filed by GSK Dec ’ 05 Ø national applications filed in Europe and New Zealand Ø US filing had to be withdrawn for technical reasons – should be resubmitted soon Ø GSK’s 2005 sales of Requip $284 m (+34%) Ø split between Parkinson’s and RLS not disclosed by GSK Ø we believe RLS >60% of Requip sales Ø mid-single digit royalty rate 22

Flutiform ™ 23

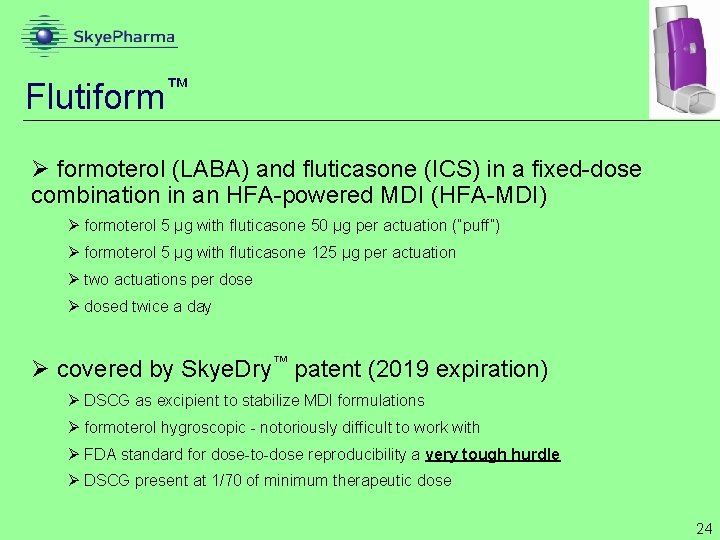
Flutiform ™ Ø formoterol (LABA) and fluticasone (ICS) in a fixed-dose combination in an HFA-powered MDI (HFA-MDI) Ø formoterol 5 μg with fluticasone 50 μg per actuation (“puff”) Ø formoterol 5 μg with fluticasone 125 μg per actuation Ø two actuations per dose Ø dosed twice a day Ø covered by Skye. Dry™ patent (2019 expiration) Ø DSCG as excipient to stabilize MDI formulations Ø formoterol hygroscopic - notoriously difficult to work with Ø FDA standard for dose-to-dose reproducibility a very tough hurdle Ø DSCG present at 1/70 of minimum therapeutic dose 24

Flutiform - launch profile “The best LABA with the best ICS” Ø formoterol LABA (long-acting beta-2 agonist) Ø 12 hours bronchodilation – twice-daily dosing Ø faster onset of action (1 -3 mins) than salmeterol (30 -45 mins) Ø gives rapid relief from common symptom of wheeziness on waking Ø patient confidence that medication is working Ø enhances compliance Ø less risk of over-dosage Ø fluticasone ICS (inhaled corticosteroid) Ø perceived to have a better safety & efficacy profile than budesonide Ø fluticasone is the physician-preferred ICS in the US Ø US physicians in particular are concerned by the risk of systemic uptake of inhaled steroids Source: TVG market research 2004 (commissioned by Skye. Pharma) 25

™ Flutiform - competitive landscape Source: Skye. Pharma 26

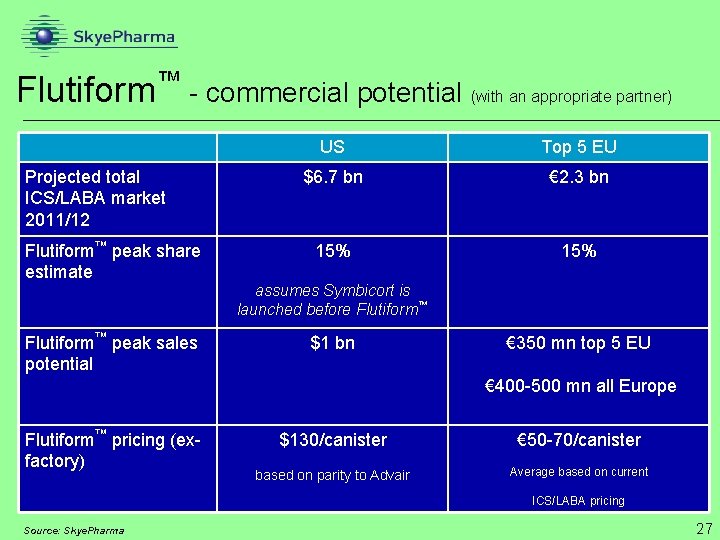
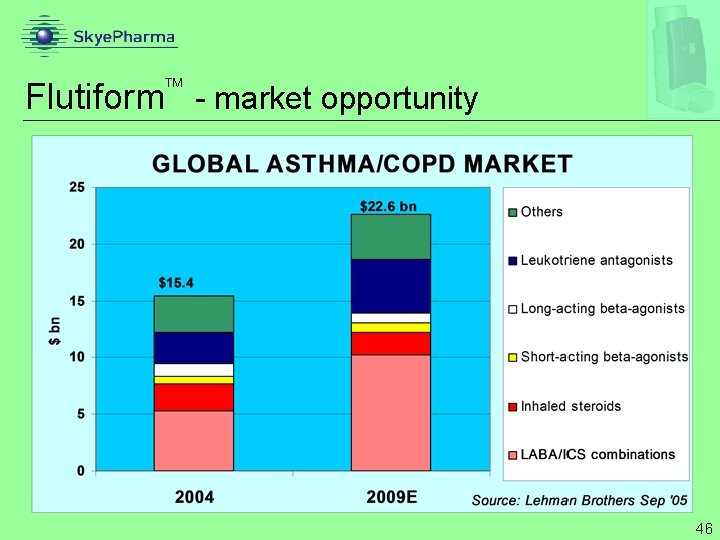
™ Flutiform - commercial potential (with an appropriate partner) Projected total ICS/LABA market 2011/12 Flutiform™ peak share estimate Flutiform™ peak sales potential US Top 5 EU $6. 7 bn € 2. 3 bn 15% assumes Symbicort is launched before Flutiform™ $1 bn € 350 mn top 5 EU € 400 -500 mn all Europe Flutiform™ pricing (exfactory) $130/canister € 50 -70/canister based on parity to Advair Average based on current ICS/LABA pricing Source: Skye. Pharma 27

™ Fluti. Form - Phase III trials Ø 3 double-blind pivotal trials Ø Flutiform™ vs two active ingredients and placebo Ø primary end-point : FEV 1 Ø patient population: mild-moderate asthmatics age >12 Ø duration of trials 12 weeks Ø one open-label 12 -month safety study Ø conducted in parallel with pivotal trials Ø total number of patients N = 1700 Ø ~300 treatment centres in Europe and North America Ø started as planned in Feb ’ 06 Ø target filing Q 3 ‘ 07 28

Injectable products and pipeline see Appendices 29

Donald Nicholson Finance Director 30

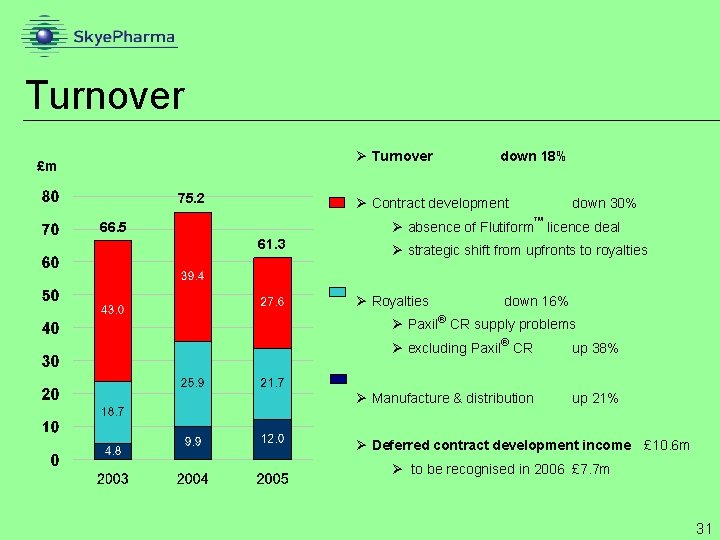
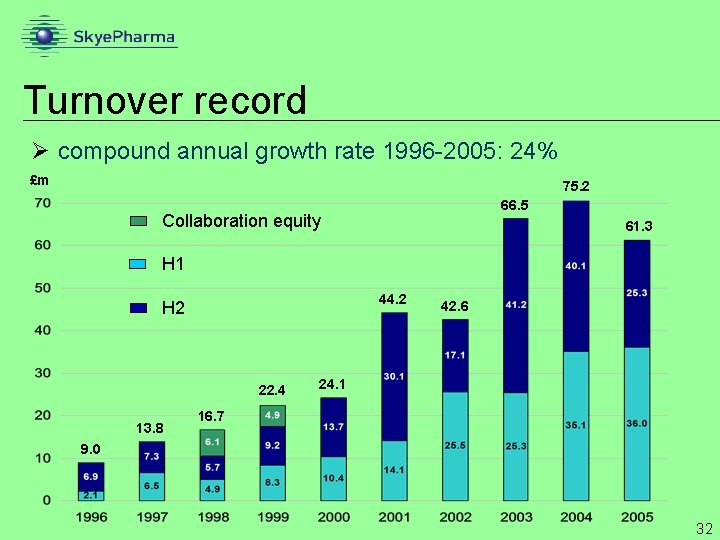
Turnover Ø Turnover £m 75. 2 down 18% Ø Contract development down 30% Ø absence of Flutiform licence deal 66. 5 61. 3 Ø strategic shift from upfronts to royalties Ø Royalties down 16% Ø Paxil CR supply problems Ø excluding Paxil CR up 38% Ø Manufacture & distribution up 21% Ø Deferred contract development income £ 10. 6 m Ø to be recognised in 2006 £ 7. 7 m 31

Turnover record Ø compound annual growth rate 1996 -2005: 24% £m 75. 2 66. 5 Collaboration equity 61. 3 H 1 44. 2 H 2 22. 4 13. 8 42. 6 24. 1 16. 7 9. 0 32

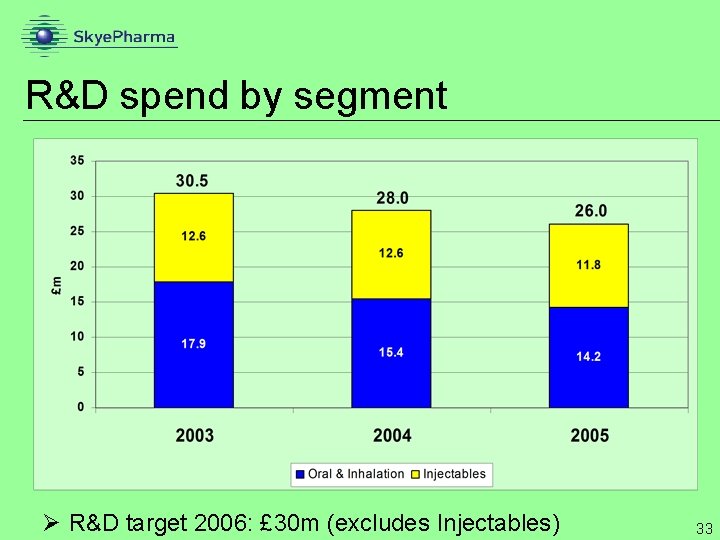
R&D spend by segment Ø R&D target 2006: £ 30 m (excludes Injectables) 33

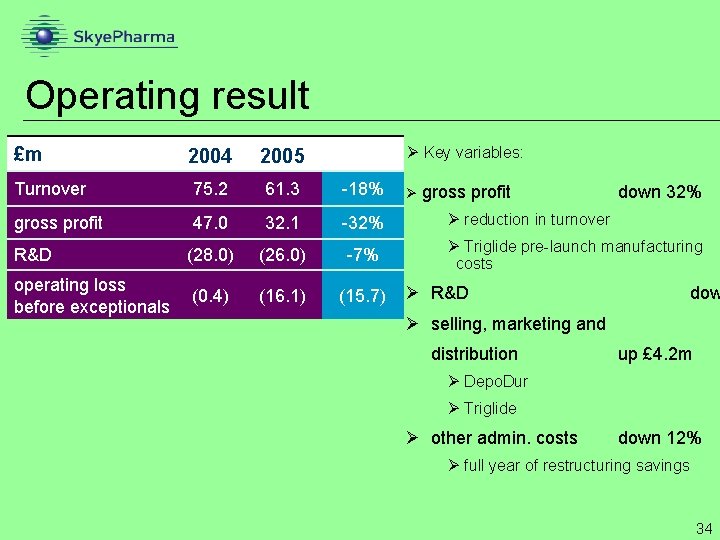
Operating result £m 2004 2005 Turnover 75. 2 61. 3 -18% gross profit 47. 0 32. 1 -32% R&D (28. 0) (26. 0) -7% operating loss before exceptionals (0. 4) (16. 1) (15. 7) Ø Key variables: Ø gross profit down 32% Ø reduction in turnover Ø Triglide pre-launch manufacturing costs Ø R&D dow Ø selling, marketing and distribution up £ 4. 2 m Ø Depo. Dur Ø Triglide Ø other admin. costs down 12% Ø full year of restructuring savings 34

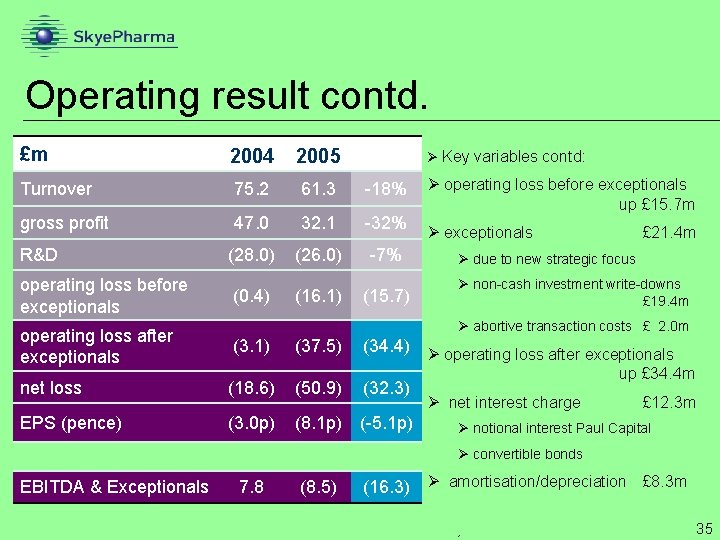
Operating result contd. £m 2004 2005 Turnover 75. 2 61. 3 -18% gross profit 47. 0 32. 1 -32% R&D (28. 0) (26. 0) -7% operating loss before exceptionals (0. 4) (16. 1) (15. 7) Ø Key variables contd: Ø operating loss before exceptionals up £ 15. 7 m Ø exceptionals £ 21. 4 m Ø due to new strategic focus Ø non-cash investment write-downs £ 19. 4 m Ø abortive transaction costs £ 2. 0 m operating loss after exceptionals (3. 1) net loss (18. 6) (50. 9) (32. 3) EPS (pence) (3. 0 p) (8. 1 p) (-5. 1 p) (37. 5) (34. 4) Ø operating loss after exceptionals up £ 34. 4 m Ø net interest charge £ 12. 3 m Ø notional interest Paul Capital Ø convertible bonds EBITDA & Exceptionals 7. 8 (8. 5) (16. 3) Ø amortisation/depreciation £ 8. 3 m Ø 35

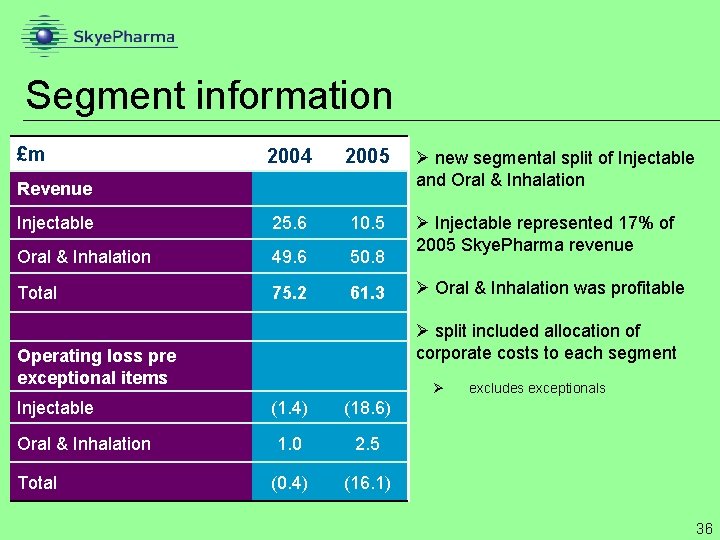
Segment information £m 2004 2005 Ø new segmental split of Injectable and Oral & Inhalation Injectable 25. 6 10. 5 Oral & Inhalation 49. 6 50. 8 Ø Injectable represented 17% of 2005 Skye. Pharma revenue Total 75. 2 61. 3 Revenue Ø split included allocation of corporate costs to each segment Operating loss pre exceptional items Injectable Oral & Inhalation Total Ø Oral & Inhalation was profitable Ø (1. 4) (18. 6) 1. 0 2. 5 (0. 4) (16. 1) excludes exceptionals 36

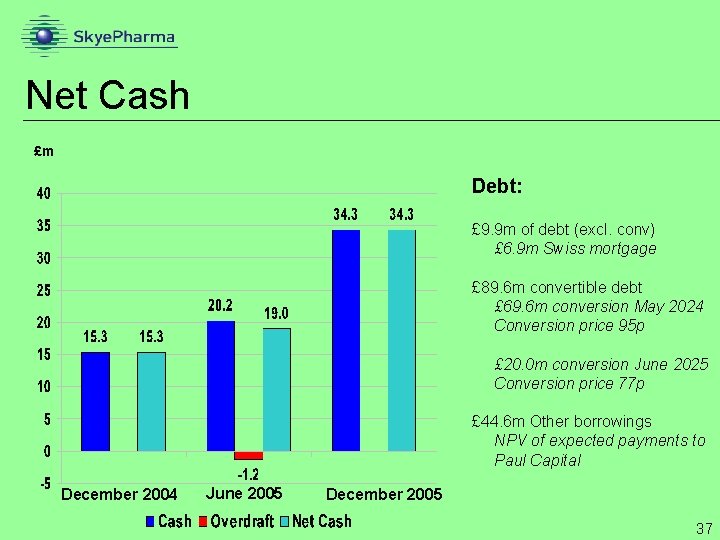
Net Cash £m Debt: £ 9. 9 m of debt (excl. conv) £ 6. 9 m Swiss mortgage £ 89. 6 m convertible debt £ 69. 6 m conversion May 2024 Conversion price 95 p £ 20. 0 m conversion June 2025 Conversion price 77 p £ 44. 6 m Other borrowings NPV of expected payments to Paul Capital December 2004 June 2005 December 2005 37

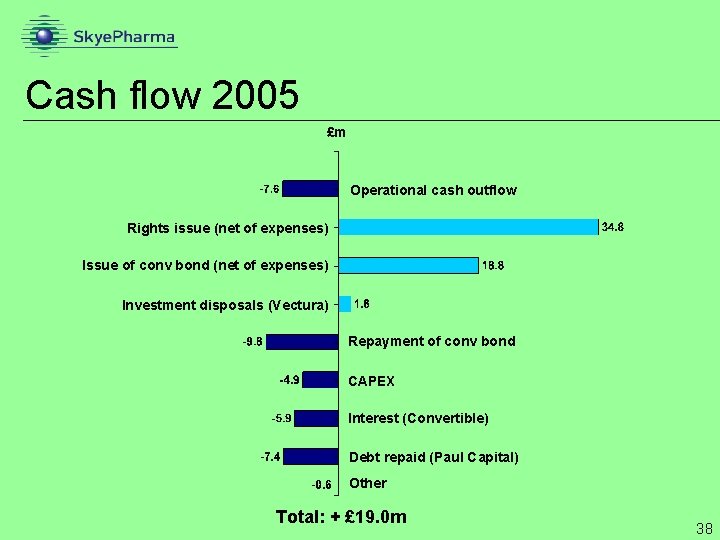
Cash flow 2005 £m Operational cash outflow Rights issue (net of expenses) Issue of conv bond (net of expenses) Investment disposals (Vectura) Repayment of conv bond CAPEX Interest (Convertible) Debt repaid (Paul Capital) Other Total: + £ 19. 0 m 38

Financial summary 2005 Ø turnover down 18% Ø royalties excluding Paxil CR up 38% Ø manufacturing & distribution up 21% Ø sustained investment in R&D £ 26 m Ø 2006 R&D target £ 30 m (excluding Injectables) Ø exceptionals £ 21. 4 m (90% non-cash write-offs) Ø key near term focus on licensing Flutiform and sale of Injectables business Ø reduce current and future cash burn and provide funds for oral and inhalation growth 39

Frank Condella Chief Executive Officer 40

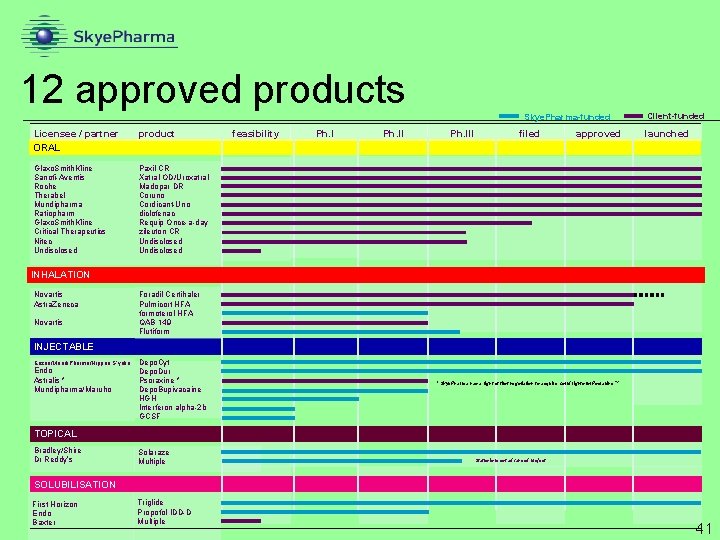
12 approved products Skye. Pharma-funded Licensee / partner ORAL product Glaxo. Smith. Kline Sanofi-Aventis Roche Therabel Mundipharma Ratiopharm Glaxo. Smith. Kline Critical Therapeutics Nitec Undisclosed Paxil CR Xatral OD/Uroxatral Madopar DR Coruno Cordicant-Uno diclofenac Requip Once-a-day zileuton CR Undisclosed feasibility Ph. III filed approved Client-funded launched INHALATION Novartis Astra. Zeneca Novartis Foradil Certihaler Pulmicort HFA formoterol HFA QAB 149 Flutiform INJECTABLE Enzon/Mundi. Pharma/Nippon S’yaku Endo Astralis * Mundipharma/Maruho Depo. Cyt Depo. Dur Psoraxine * Depo. Bupivacaine HGH Interferon alpha-2 b GCSF * Skye. Pharma has a right of first negotiation to acquire world rights for Psoraxine. TM TOPICAL Bradley/Shire Dr Reddy’s Solaraze Multiple Status is most advanced project SOLUBILISATION First Horizon Endo Baxter Triglide Propofol IDD-D Multiple 41

Major objectives 2006 Ø divest Injectables unit Ø outlicense Flutiform™ Ø complete modifications to Certihaler™ / relaunch in EU Ø expand oral / inhalation pipeline Ø work with licensing partners to drive revenues of marketed products 42

The new Skye. Pharma Ø new leadership Ø drive for sustainable profitability Ø accelerated by sale of injectables unit Ø core business is oral and inhalation Ø potential blockbuster in Flutiform™ Ø strong cashflow from existing royalties 43

Investor relations contacts: London Peter Laing, Director of Corporate Communications 44 -(0)207 -491 -1777 plaing@skyepharma. co. uk New York Sandra Haughton, US Investor Relations Manager 1 -212 -753 -5780 shaughton@skyepharma. com …and please visit our website NASDAQ: SKYE LSE: SKP www. skyepharma. com

Appendices 45

Flutiform - market opportunity 46

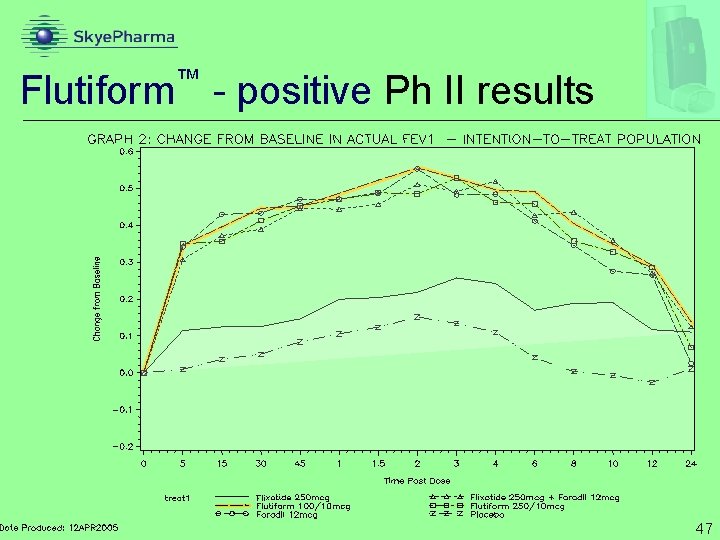
™ Flutiform - positive Ph II results 47

Injectables business 48

Major marketed products Injectable: Depo. Cyt (Enzon; Mundipharma) Depo. Dur (Endo) 49

Depo. Cyt Enzon / Mundipharma Ø intrathecal injectable cytarabine for lymphomatous meningitis Ø severe complication of lymphoma Ø Depofoam formulation extends period between injections from 1 -2 days to 2 weeks Ø treatment can be on out-patient basis Ø marketed by Enzon in US, Mundipharma in Europe (as Depo. Cyte ) Ø 2005 global in-market sales $14 m (+75%) Ø US sales $8 m (+26%) Ø Europe sales $6 m (vs $1. 5 m in 2004, launch year) Ø Ph IV trial in solid tumour patients now completed Ø most common type of neoplastic meningitis Ø expect to file data with FDA shortly Ø filed in Europe – response due mid-06 Ø Skye. Pharma share of sales: 35% 50

Depo. Dur Endo Ø sustained–release injectable morphine for relief of post-operative pain Ø given as single epidural injection during/after surgery Ø provides effective relief of pain for 48 hours after surgery (period of peak pain) Ø avoids catheter-related problems with conventional short-acting morphine Ø potential to replace conventional infusion pumps (major source of equipment failure) Ø USA (Endo Pharmaceuticals) Ø launched by Endo Dec ‘ 04 – hospital sales force 70 targeting 1000 key hospitals Ø some initial shipment problems Ø formulary acceptance has taken much longer than expected Ø on ~50% of hospital formularies by end-05 – “still in launch phase” Ø 2005 sales $4 m Ø Europe Ø UK marketing authorisation process has been protracted but approval imminent Ø once approved in UK, approvals in other EU countries to follow over next 6 -12 months Ø Zeneus acquired by Cephalon – European rights have reverted to Skye. Pharma 51

Key pipeline products Injectable: Depo. Bupivacaine Biologics 52

Depo. Bupivacaine Mundipharma/Maruho Ø long-acting injectable formulation of local anaesthetic Ø designed to provide 48 -72 hours of local pain relief after out-patient surgery Ø complementary with Depo. Dur (for in-patient surgery) Ø Ph II trials completed Ø Ph III trial to commence mid ‘ 06 Ø licensed to Mundipharma outside North America/Japan Apr ’ 05 Ø $80 m in milestones + 35% share of sales (30% outside Europe) Ø licensed to Maruho for Japan Jul ’ 05 Ø $18 m in milestones + undisclosed share of sales Ø Maruho funds development for Japanese approval Ø Endo had right of first negotiation for North America after Ph II Ø decided not to exercise – simplifies divestment of injectables unit Ø Skye. Pharma will aim to retain economic interest in Depo. Bupivacaine 53

Biologics Ø two complementary sustained-release injectable technologies: Depo. Foam™ and Biosphere™ Ø both suitable for proteins and other macromolecules Ø mild process conditions help conserve native protein conformation Ø injection frequency can be tailored in range 7 - 28 days Ø Skye. Pharma has now successfully formulated seven different protein drugs Ø includes G-CSF, EPO, h. GH, IFN-α and IFN-β Ø in 2005 three new feasibility study agreements with partners Ø several projects to enter Phase I clinical trials in 2007 54

Propofol IDD-D Endo Ø proprietary improved version of propofol Ø Astra. Zeneca’s Diprivan - injectable anaesthetic and sedative Ø uses Skye. Pharma’s proprietary solubilisation technology to stabilise emulsion Ø avoids need for an antimicrobial preservative Ø designed for continuous uninterrupted 24 -hour sedation Ø completed Ph II trials late 2004 Ø still in discussion with FDA on design of Ph III trials! 55
 Chapter 9 lesson 3 commander in chief and chief diplomat
Chapter 9 lesson 3 commander in chief and chief diplomat Chief executive power
Chief executive power Chief executive power
Chief executive power Gavin haynes camden
Gavin haynes camden Capitalize chief executive officer
Capitalize chief executive officer Chief executive powers
Chief executive powers Torfaen council chief executive
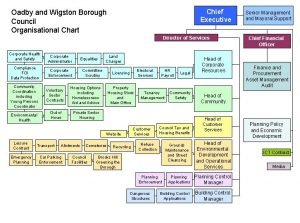
Torfaen council chief executive Oadby and wigston refuse tip
Oadby and wigston refuse tip Chief executive boards international
Chief executive boards international 2006 calendar year
2006 calendar year The results of dr.frank's experiment
The results of dr.frank's experiment Frank william abagnale sr
Frank william abagnale sr Brigance 2 year old data sheet
Brigance 2 year old data sheet Poems for leavers assembly
Poems for leavers assembly Chief knowledge officer
Chief knowledge officer Chief black kettle
Chief black kettle Chief tomochichi
Chief tomochichi In scouts eyes what is atticus chief fault
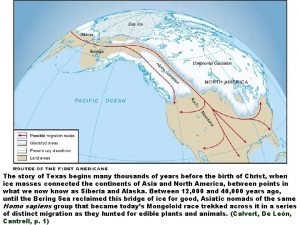
In scouts eyes what is atticus chief fault My story is one of many thousands
My story is one of many thousands The snake chief a xhosa tale
The snake chief a xhosa tale The ninth commandment catholic
The ninth commandment catholic The ransom of red chief questions
The ransom of red chief questions Chief sohcahtoa story
Chief sohcahtoa story George w bush father
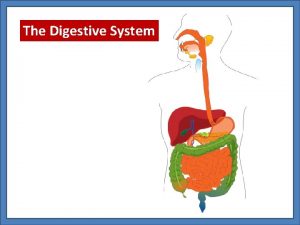
George w bush father Chief factory for digestive enzymes
Chief factory for digestive enzymes Chief data officer training
Chief data officer training Ransom of red chief plot diagram
Ransom of red chief plot diagram Chief servant meaning
Chief servant meaning Clique vs crowd
Clique vs crowd The chief determinant of popularity in high school is
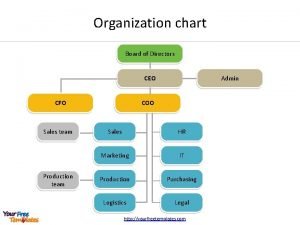
The chief determinant of popularity in high school is Ceo coo organizational chart
Ceo coo organizational chart Principal chief controller of accounts
Principal chief controller of accounts Chief diplomat def
Chief diplomat def Pqrstu mnemonic
Pqrstu mnemonic 6 chief parts of the catechism
6 chief parts of the catechism Alcock v chief constable of south yorkshire
Alcock v chief constable of south yorkshire Cuckoo quotes
Cuckoo quotes Hfd chief login
Hfd chief login Chief network architect
Chief network architect What covers the trachea when swallowing
What covers the trachea when swallowing Where are chief cells located
Where are chief cells located Chief technology strategist
Chief technology strategist Cc hpi pmh
Cc hpi pmh Chapter 14 the presidency in action answer key
Chapter 14 the presidency in action answer key Chief instructional officers california community colleges
Chief instructional officers california community colleges California community college chief instructional officers
California community college chief instructional officers Yaru ancient egypt
Yaru ancient egypt Cc and hpi
Cc and hpi Uk chief medical officers' physical activity guidelines
Uk chief medical officers' physical activity guidelines Who is the chief guest at macbeth's celebration feast
Who is the chief guest at macbeth's celebration feast The ransom of red chief vocabulary
The ransom of red chief vocabulary The ransom of red chief theme
The ransom of red chief theme First 90 days as chief of staff
First 90 days as chief of staff Chief scientist scotland
Chief scientist scotland The chief cornerstone high school
The chief cornerstone high school Massacre midge
Massacre midge