What would happen if you put a dry









- Slides: 20

What would happen if you put a dry sponge under a trickling faucet?

The sponge would absorb the water until at a certain point water would start to drip from it. You could say that the sponge had a water capacity: It could hold so much water before it couldn’t hold any more and the water started dripping out.

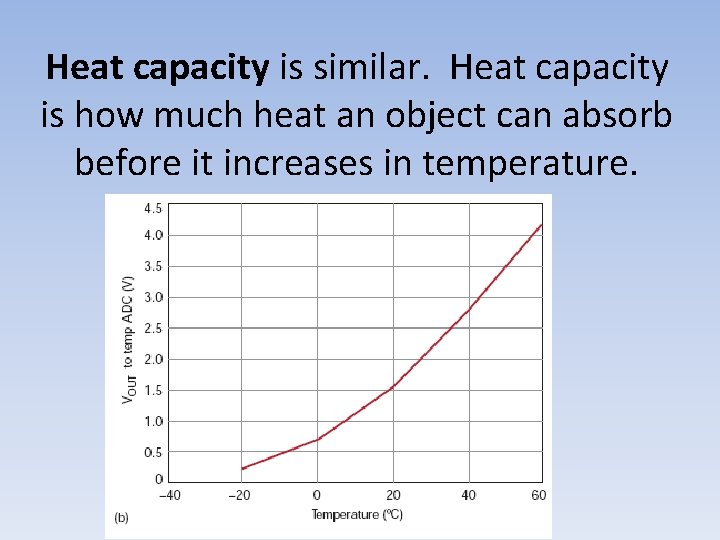
Heat capacity is similar. Heat capacity is how much heat an object can absorb before it increases in temperature.

The mass of an object influences its heat capacity (like a large sponge can hold more water than a smaller sponge), and so does the material itself. Think of putting a piece of wood under a faucet. How would this compare to the sponge?

The water would begin to drip out sooner, right? It wouldn’t have much water capacity compared to a sponge. Similarly, different types of substances have different capacities for absorbing heat.

Specific Heat Specific heat is how much heat energy a single gram of material must absorb before it increases 1°C. Each material has its own specific heat. The higher a material’s specific heat, the more heat it must absorb before it increases in temperature.

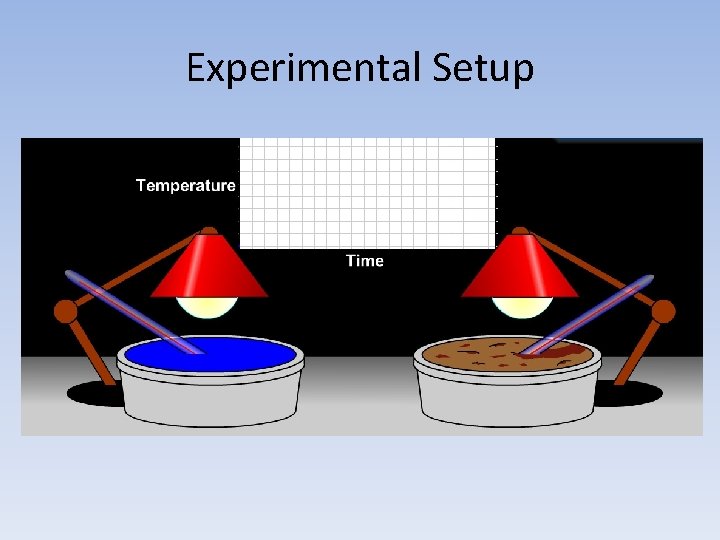
Experimental Setup

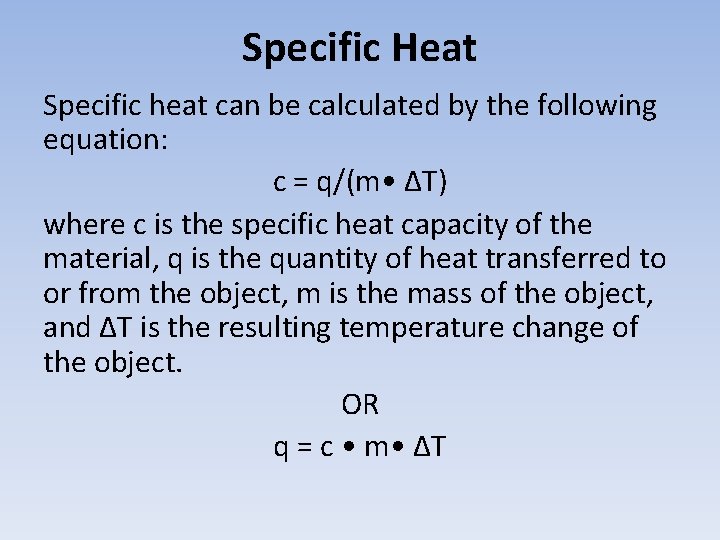
Specific Heat Specific heat can be calculated by the following equation: c = q/(m • ΔT) where c is the specific heat capacity of the material, q is the quantity of heat transferred to or from the object, m is the mass of the object, and ΔT is the resulting temperature change of the object. OR q = c • m • ΔT

O 2 Dissolved Oxygen O 2 Oxygen is a soluble gas! O 2 O 2 O 2 Oxygen in water usually enters the water from the air at the surface, where it slowly dissolves. The oxygen then diffuses in the water, very slowlyabout 300, 000 times more slowly than it does in air.

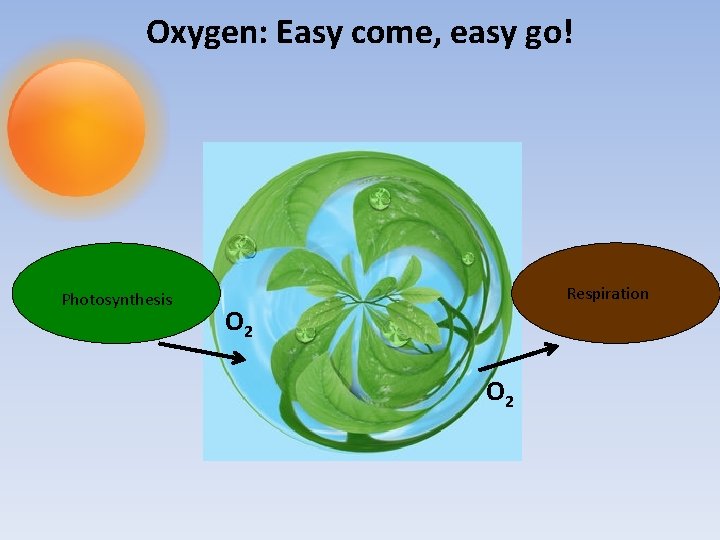
Oxygen: Easy come, easy go! Photosynthesis Respiration O 2

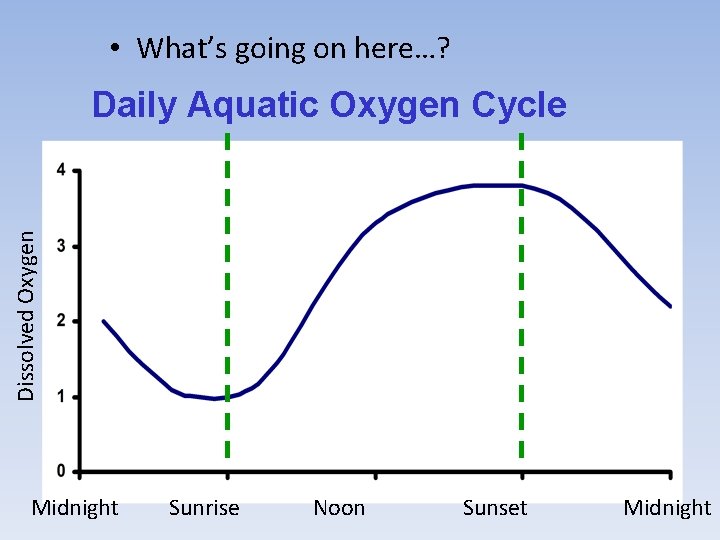
• What’s going on here…? Dissolved Oxygen Daily Aquatic Oxygen Cycle Midnight Sunrise Noon Sunset Midnight

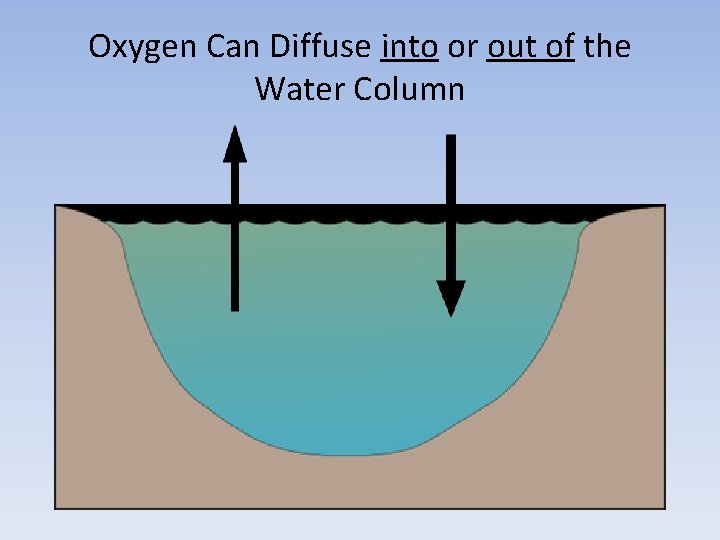
Oxygen Can Diffuse into or out of the Water Column

The air is 21% oxygen O 2 O 2 O 2 OWater holds much less than 1% 2 O 2 O 2 O O 2 2 O 2 O 2 O 2

Hmmm… • How do you think oxygen might get into the water from the air? • What kinds of factors might affect the amount of oxygen in the water? • Do you think the temperature of water can affect the amount of oxygen in it? If so, how? • Would you predict warm water to have more oxygen or less than cold water?

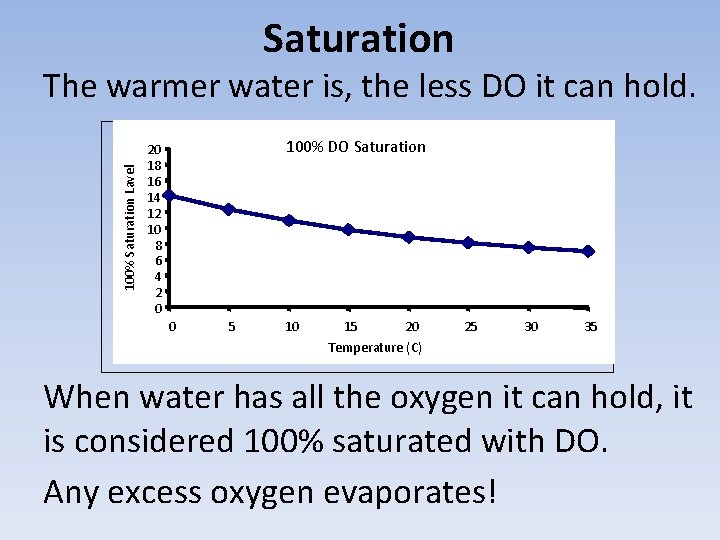
Saturation 100% Saturation Lavel The warmer water is, the less DO it can hold. 100% DO Saturation 20 18 16 14 12 10 8 6 4 2 0 0 5 10 15 20 25 30 35 Temperature (C) When water has all the oxygen it can hold, it is considered 100% saturated with DO. Any excess oxygen evaporates!

Why does it matter? For mg/L (or ppm): • 0 -2 mg/L: not enough oxygen to support most animals • 2 -4 mg/L: only a few kinds of fish and insects can survive • 4 -7 mg/L: good for most kinds of pond animals • 7 -11 mg/L: very good for most stream fish For percent saturation: • Below 60%: poor quality, bacteria may be using up DO • 60 -79%: acceptable for most stream animals • 80 -125%: excellent for most stream animals • 125% or more: too high

Instream Dissolved Oxygen 11 9 8 7 6 8 6 5 4 3 8 5 4 SALMONID WATERS A. Embryo and larval stages No production impairment Slight production impairment Moderate production impairment Severe production impairment Limit to avoid acute mortality B. Other life stages No production impairment Slight production impairment Moderate production impairment Severe production impairment Limit to avoid acute mortality III. INVERTEBRATES No production impairment Moderate production impairment Limit to avoid acute mortality

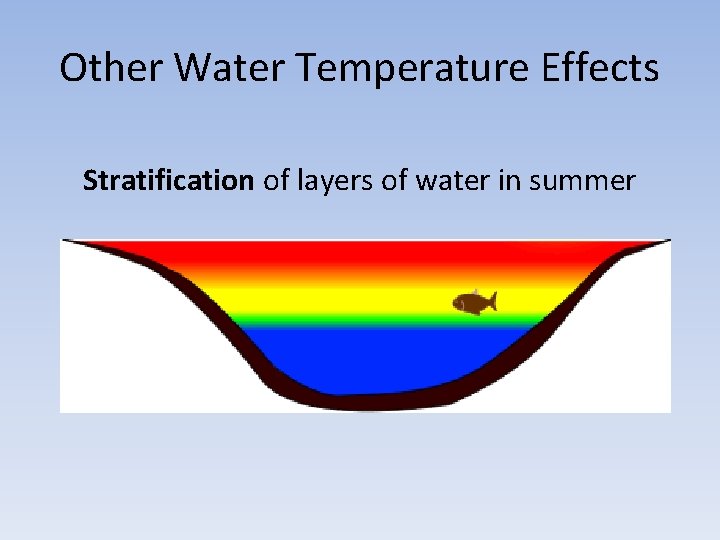
Other Water Temperature Effects Stratification of layers of water in summer

Ice cover as water cools to freezing

Growth and survival of aquatic organisms