WAVE MECHANICS Schrdinger 1926 The currently accepted version




- Slides: 10

WAVE MECHANICS (Schrödinger, 1926) The currently accepted version of quantum mechanics which takes into account the wave nature of matter and the uncertainty principle. * The state of an electron is described by a function y, called the “wave function”. * y can be obtained by solving Schrödinger’s equation (a differential equation): Hy=Ey ^ This equation can be solved exactly only for the H atom

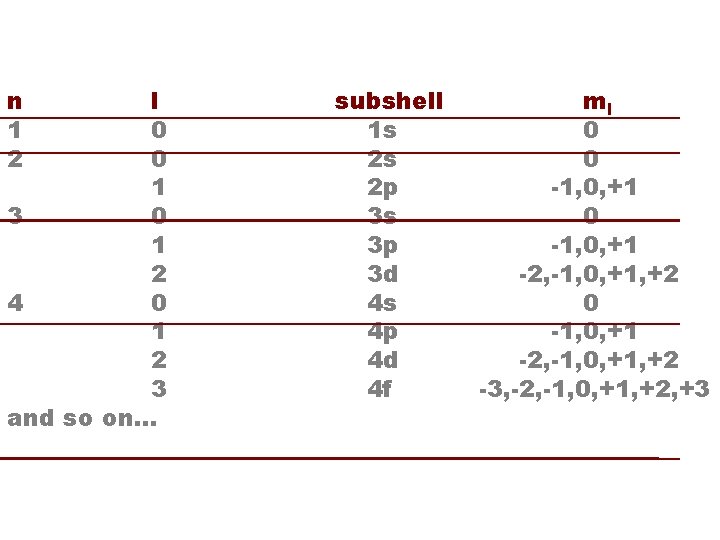
WAVE MECHANICS * This equation has multiple solutions (“orbitals”), each corresponding to a different energy level. * Each orbital is characterized by three quantum numbers: n : principal quantum number n=1, 2, 3, . . . l : azimuthal quantum number l= 0, 1, …n-1 ml: magnetic quantum number ml= -l, …, +l

WAVE MECHANICS * The energy depends only on the principal quantum number, as in the Bohr model: En = -2. 179 X 10 -18 J /n 2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0, 1, 2, 3, 4, 5, . . . s p d f g h. . . * All orbitals with the same n are called a “shell”. All orbitals with the same n and l are called a “subshell”.

HYDROGEN ORBITALS n 1 2 l 0 0 1 3 0 1 2 4 0 1 2 3 and so on. . . subshell 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f ml 0 0 -1, 0, +1 -2, -1, 0, +1, +2 -3, -2, -1, 0, +1, +2, +3

What is the physical meaning of the wave function? BORN POSTULATE The probability of finding an electron in a certain region of space is proportional to y 2, the square of the value of the wavefunction at that region. y can be positive or negative. y 2 is always positive y 2 is called the “electron density”

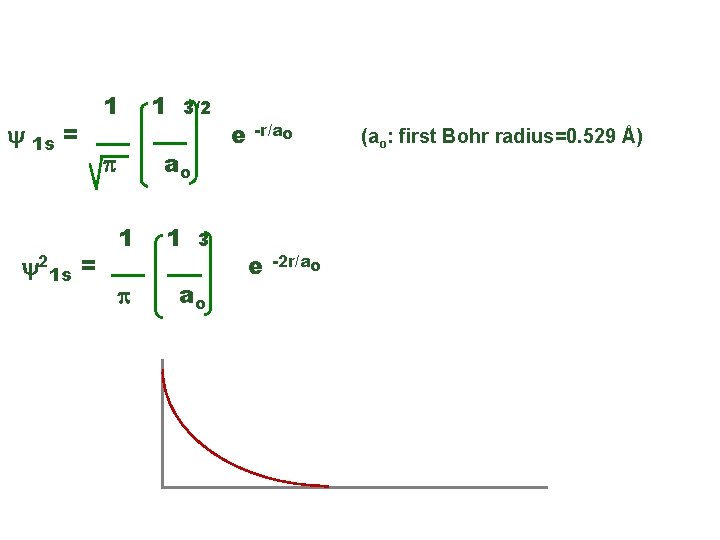
E. g. , the hydrogen ground state y 1 s = y 21 s = 1 1 3/2 ao p 1 3 ao e -r/ao e (ao: first Bohr radius=0. 529 Å) -2 r/ao y 21 s r

Higher s orbitals All s orbitals are spherically symmetric

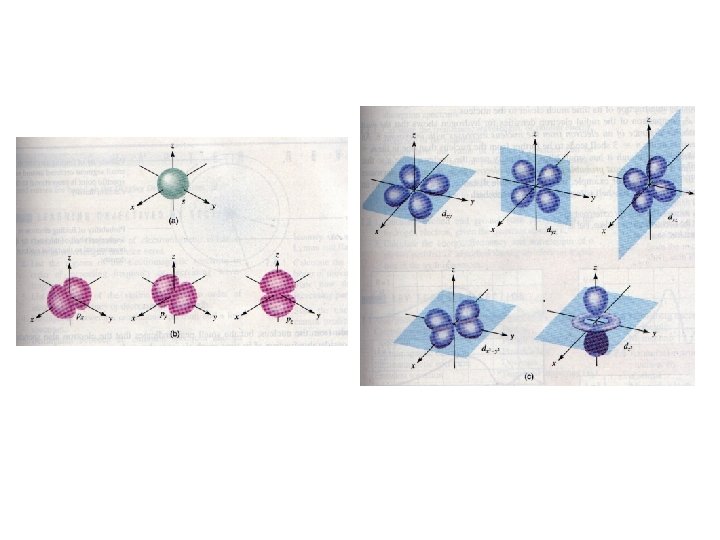
Balloon pictures of orbitals The shape of the orbital is determined by the l quantum number. Its orientation by ml.

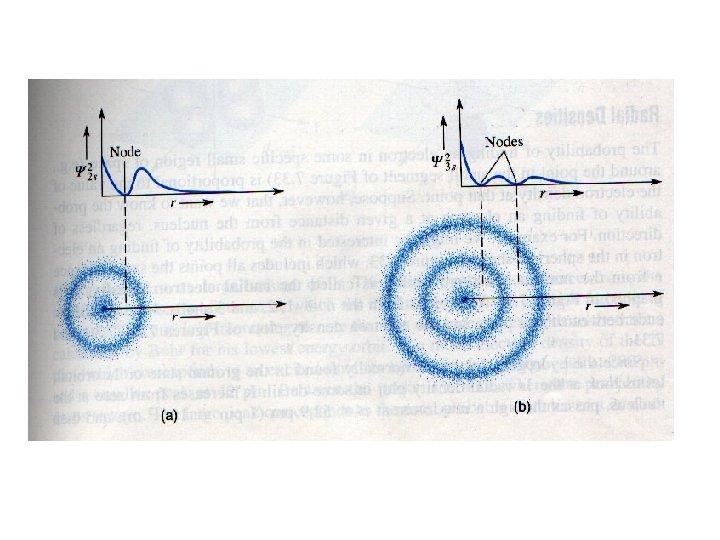
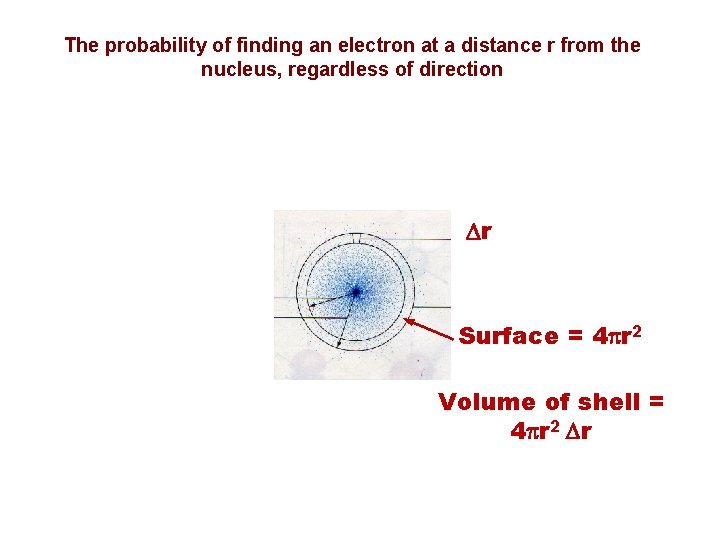
Radial electron densities The probability of finding an electron at a distance r from the nucleus, regardless of direction The radial electron density is proportional to r 2 y 2 Dr Surface = 4 pr 2 Volume of shell = 4 pr 2 Dr

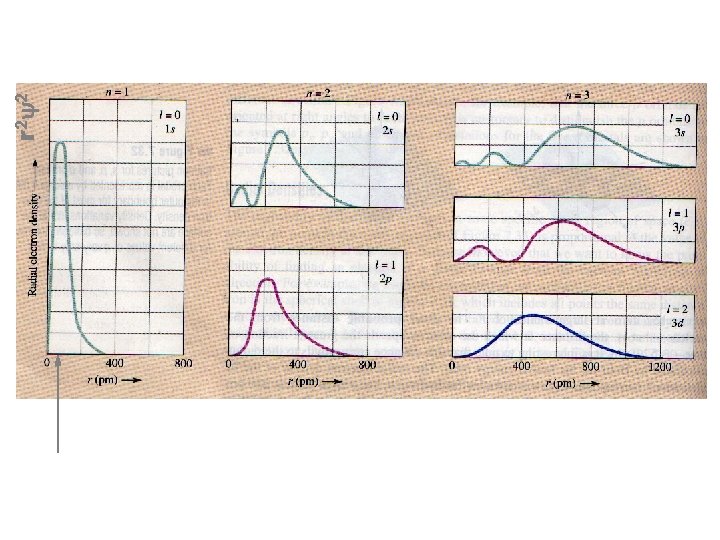
r 2 y 2 Radial electron densities Maximum here corresponds to the first Bohr radius