VK 2809 in NAFLD a phase 2 study
- Slides: 7

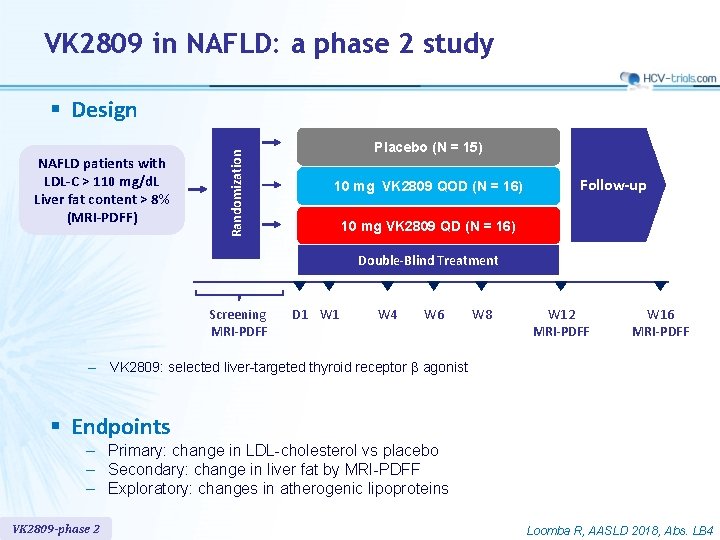
VK 2809 in NAFLD: a phase 2 study NAFLD patients with LDL-C > 110 mg/d. L Liver fat content > 8% (MRI-PDFF) Randomization § Design Placebo (N = 15) 10 mg VK 2809 QOD (N = 16) Follow-up 10 mg VK 2809 QD (N = 16) Double-Blind Treatment Screening MRI-PDFF D 1 W 4 W 6 W 8 W 12 MRI-PDFF W 16 MRI-PDFF ‒ VK 2809: selected liver-targeted thyroid receptor β agonist § Endpoints – Primary: change in LDL-cholesterol vs placebo – Secondary: change in liver fat by MRI-PDFF – Exploratory: changes in atherogenic lipoproteins VK 2809 -phase 2 Loomba R, AASLD 2018, Abs. LB 4

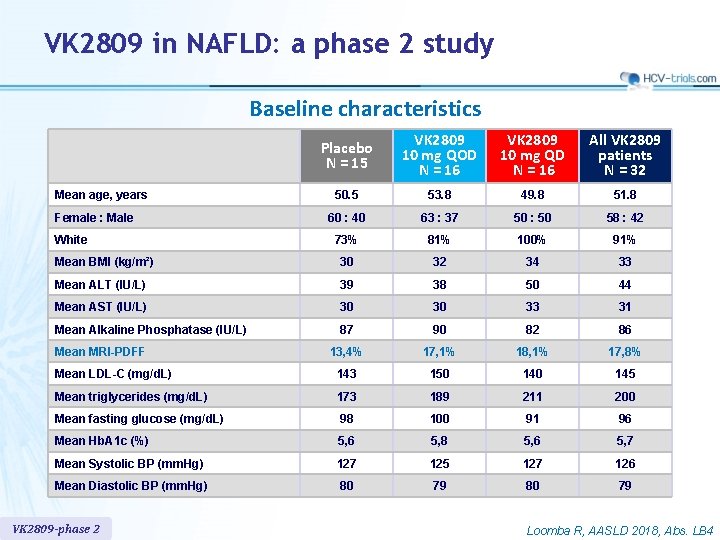
VK 2809 in NAFLD: a phase 2 study Baseline characteristics Placebo N = 15 VK 2809 10 mg QOD N = 16 VK 2809 10 mg QD N = 16 All VK 2809 patients N = 32 50. 5 53. 8 49. 8 51. 8 60 : 40 63 : 37 50 : 50 58 : 42 73% 81% 100% 91% Mean BMI (kg/m²) 30 32 34 33 Mean ALT (IU/L) 39 38 50 44 Mean AST (IU/L) 30 30 33 31 Mean Alkaline Phosphatase (IU/L) 87 90 82 86 13, 4% 17, 1% 18, 1% 17, 8% Mean LDL-C (mg/d. L) 143 150 145 Mean triglycerides (mg/d. L) 173 189 211 200 Mean fasting glucose (mg/d. L) 98 100 91 96 Mean Hb. A 1 c (%) 5, 6 5, 8 5, 6 5, 7 Mean Systolic BP (mm. Hg) 127 125 127 126 Mean Diastolic BP (mm. Hg) 80 79 Mean age, years Female : Male White Mean MRI-PDFF VK 2809 -phase 2 Loomba R, AASLD 2018, Abs. LB 4

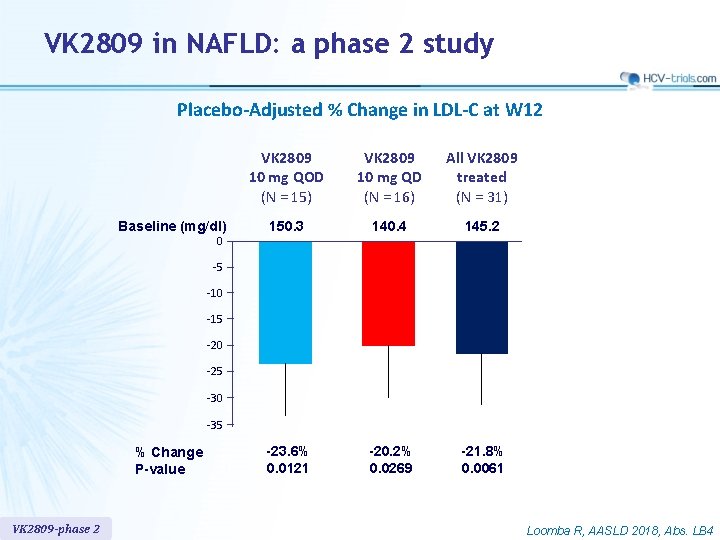
VK 2809 in NAFLD: a phase 2 study Placebo-Adjusted % Change in LDL-C at W 12 Baseline (mg/dl) 0 VK 2809 10 mg QOD (N = 15) VK 2809 10 mg QD (N = 16) All VK 2809 treated (N = 31) 150. 3 140. 4 145. 2 -23. 6% 0. 0121 -20. 2% 0. 0269 -21. 8% 0. 0061 -5 -10 -15 -20 -25 -30 -35 % Change P-value VK 2809 -phase 2 Loomba R, AASLD 2018, Abs. LB 4

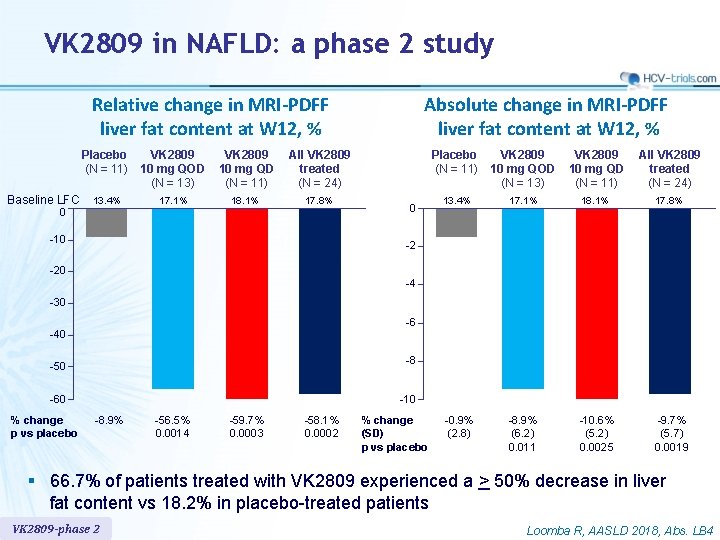
VK 2809 in NAFLD: a phase 2 study Relative change in MRI-PDFF liver fat content at W 12, % Baseline LFC Placebo (N = 11) VK 2809 10 mg QOD (N = 13) VK 2809 10 mg QD (N = 11) All VK 2809 treated (N = 24) 13. 4% 17. 1% 18. 1% 17. 8% 0 -10 Absolute change in MRI-PDFF liver fat content at W 12, % 0 Placebo (N = 11) VK 2809 10 mg QOD (N = 13) VK 2809 10 mg QD (N = 11) All VK 2809 treated (N = 24) 13. 4% 17. 1% 18. 1% 17. 8% -0. 9% (2. 8) -8. 9% (6. 2) 0. 011 -10. 6% (5. 2) 0. 0025 -9. 7% (5. 7) 0. 0019 -2 -20 -4 -30 -6 -40 -50 -8 -60 -10 % change p vs placebo -8. 9% -56. 5% 0. 0014 -59. 7% 0. 0003 -58. 1% 0. 0002 % change (SD) p vs placebo § 66. 7% of patients treated with VK 2809 experienced a > 50% decrease in liver fat content vs 18. 2% in placebo-treated patients VK 2809 -phase 2 Loomba R, AASLD 2018, Abs. LB 4

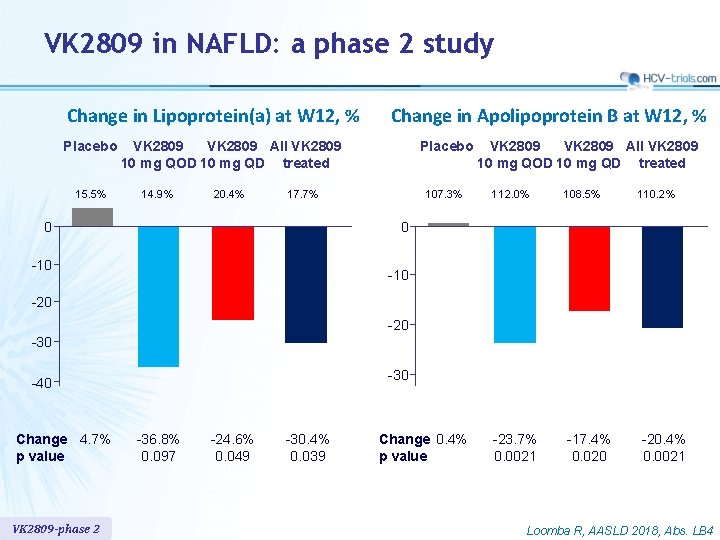
VK 2809 in NAFLD: a phase 2 study Change in Lipoprotein(a) at W 12, % Placebo 15. 5% Change in Apolipoprotein B at W 12, % VK 2809 All VK 2809 10 mg QOD 10 mg QD treated 14. 9% 20. 4% Placebo 17. 7% 0 107. 3% VK 2809 All VK 2809 10 mg QOD 10 mg QD treated 112. 0% 108. 5% -23. 7% 0. 0021 -17. 4% 0. 020 110. 2% 0 -10 -20 -30 -40 Change 4. 7% p value VK 2809 -phase 2 -36. 8% 0. 097 -24. 6% 0. 049 -30. 4% 0. 039 Change 0. 4% p value -20. 4% 0. 0021 Loomba R, AASLD 2018, Abs. LB 4

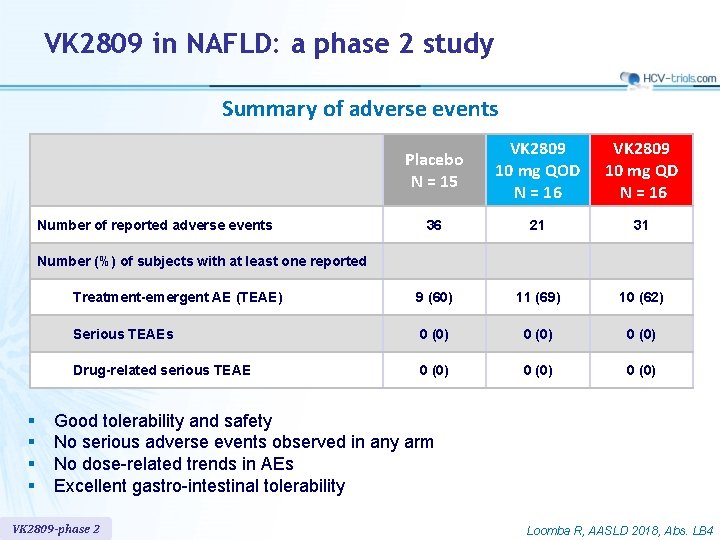
VK 2809 in NAFLD: a phase 2 study Summary of adverse events Placebo N = 15 VK 2809 10 mg QOD N = 16 VK 2809 10 mg QD N = 16 36 21 31 Treatment-emergent AE (TEAE) 9 (60) 11 (69) 10 (62) Serious TEAEs 0 (0) Drug-related serious TEAE 0 (0) Number of reported adverse events Number (%) of subjects with at least one reported § § Good tolerability and safety No serious adverse events observed in any arm No dose-related trends in AEs Excellent gastro-intestinal tolerability VK 2809 -phase 2 Loomba R, AASLD 2018, Abs. LB 4

VK 2809 in NAFLD: a phase 2 study § Summary – VK 2809 produced robust reduction in liver fat on MRI-PDFF in NAFLD patients after 12 weeks of oral dosing – Up to 91% of patients dosed with VK 2809 experienced a response as demonstrated by liver fat reductions ≥ 30% relative to baseline ; 67% experienced liver fat reductions ≥ 50% – VK 2809 produced significant reduction in LDL-C, triglycerides, Apo B, and Lp(a) relative to placebo in NAFLD patients – VK 2809 was safe and well-tolerated VK 2809 -phase 2 Loomba R, AASLD 2018, Abs. LB 4