Use of POC technologies for early diagnosis of




- Slides: 11

Use of POC technologies for early diagnosis of HIV Francesca Celletti

What is innovation? Innovation is not only development of a product. It has to bring better values for clients and return from investments. The nature of the innovation should be of definable value, introduce a concrete change and bring about a related and measurable outcome

Progress toward the target 57 -8182

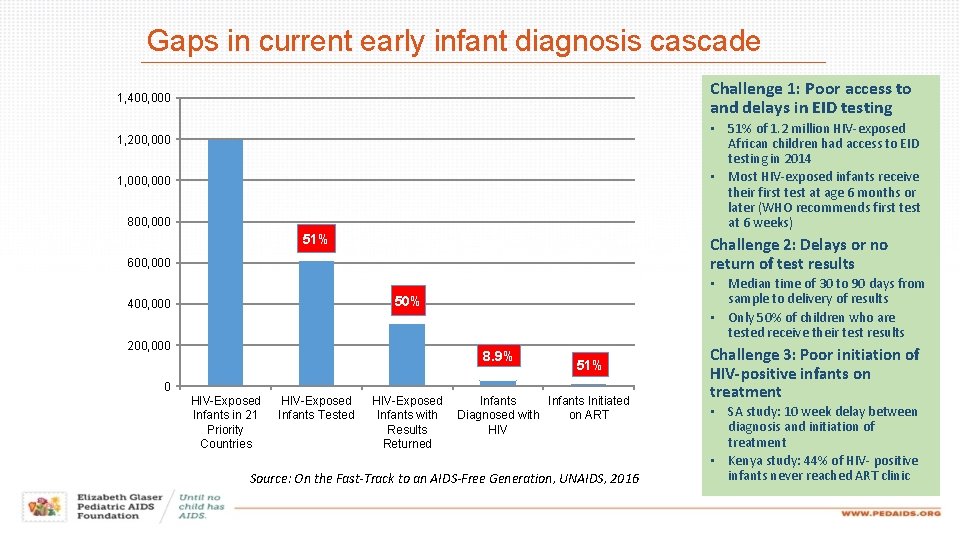
Gaps in current early infant diagnosis cascade Challenge 1: Poor access to and delays in EID testing 1, 400, 000 • 51% of 1. 2 million HIV-exposed African children had access to EID testing in 2014 • Most HIV-exposed infants receive their first test at age 6 months or later (WHO recommends first test at 6 weeks) 1, 200, 000 1, 000 800, 000 51% Challenge 2: Delays or no return of test results 600, 000 • Median time of 30 to 90 days from sample to delivery of results • Only 50% of children who are tested receive their test results 50% 400, 000 200, 000 8. 9% 51% 0 HIV-Exposed Infants in 21 Priority Countries HIV-Exposed Infants Tested HIV-Exposed Infants with Results Returned Infants Initiated Diagnosed with on ART HIV Source: On the Fast-Track to an AIDS-Free Generation, UNAIDS, 2016 Challenge 3: Poor initiation of HIV-positive infants on treatment • SA study: 10 week delay between diagnosis and initiation of treatment • Kenya study: 44% of HIV- positive infants never reached ART clinic

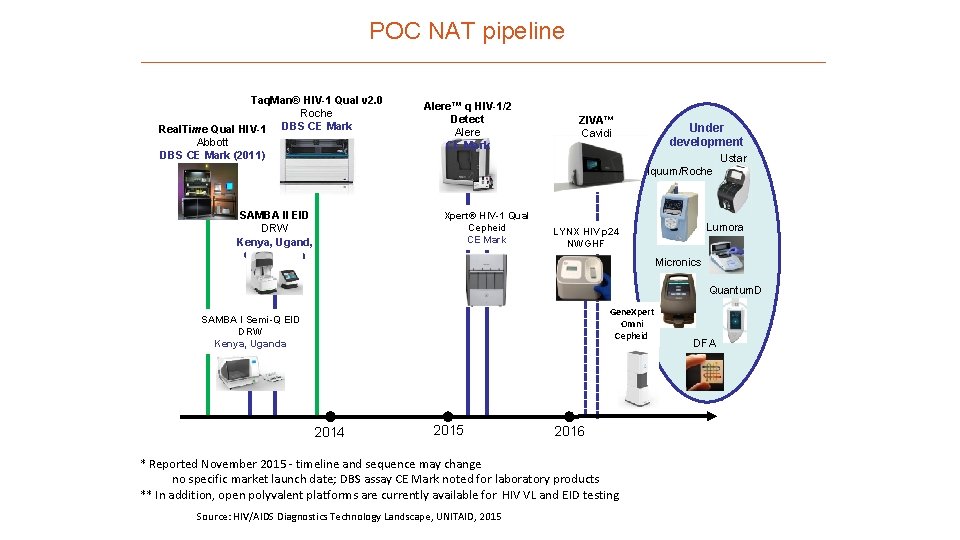
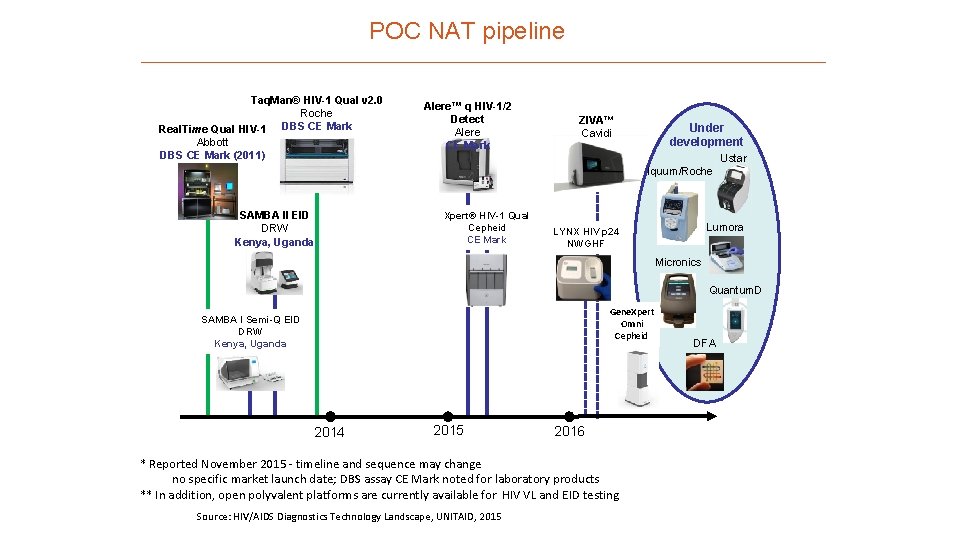
POC NAT pipeline Taq. Man® HIV-1 Qual v 2. 0 Roche DBS CE Mark Real. Time Qual HIV-1 Abbott DBS CE Mark (2011) Alere™ q HIV-1/2 Detect Alere CE Mark ZIVA™ Cavidi Under development Ustar Iquum/Roche SAMBA II EID DRW Kenya, Ugand, CE Markeda Xpert® HIV-1 Qual Cepheid CE Mark Lumora LYNX HIV p 24 NWGHF Micronics Gene. Xpert Omni Cepheid SAMBA I Semi-Q EID DRW Kenya, Uganda 2014 2015 2016 * Reported November 2015 - timeline and sequence may change no specific market launch date; DBS assay CE Mark noted for laboratory products ** In addition, open polyvalent platforms are currently available for HIV VL and EID testing Source: HIV/AIDS Diagnostics Technology Landscape, UNITAID, 2015 Quantum. D x DFA

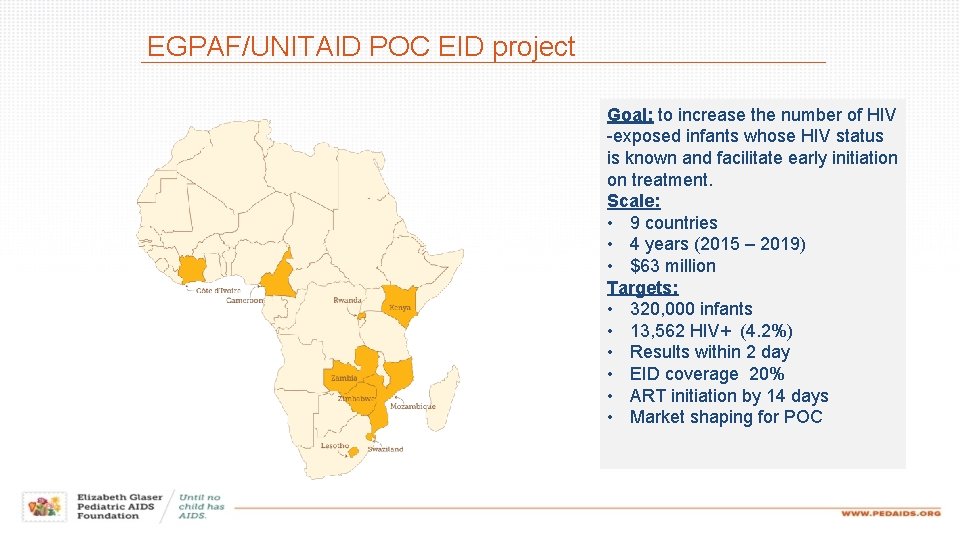
EGPAF/UNITAID POC EID project Goal: to increase the number of HIV -exposed infants whose HIV status is known and facilitate early initiation on treatment. Scale: • 9 countries • 4 years (2015 – 2019) • $63 million Targets: • 320, 000 infants • 13, 562 HIV+ (4. 2%) • Results within 2 day • EID coverage 20% • ART initiation by 14 days • Market shaping for POC

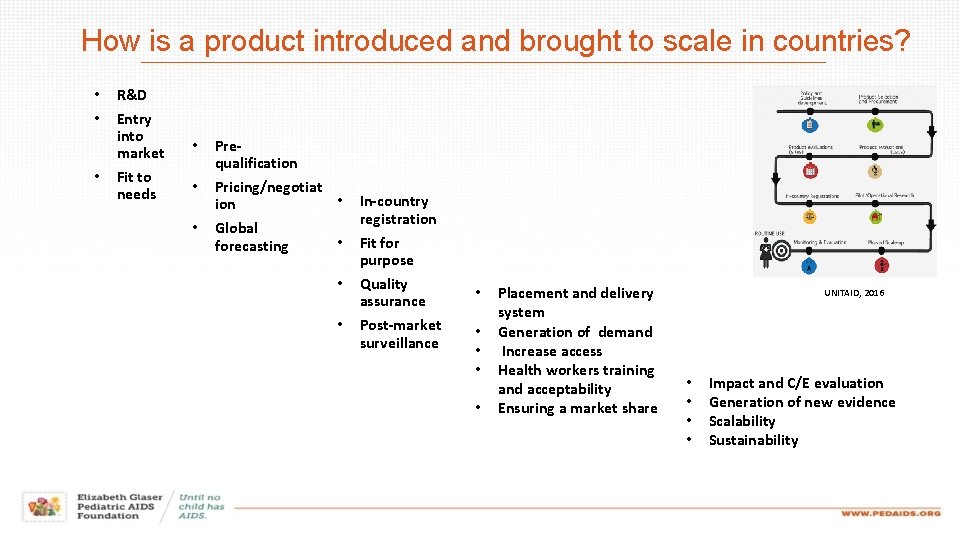
How is a product introduced and brought to scale in countries? • • • R&D Entry into market Fit to needs • Prequalification • Pricing/negotiat • ion Global • forecasting • • • In-country registration Fit for purpose Quality assurance Post-market surveillance • • • Placement and delivery system Generation of demand Increase access Health workers training and acceptability Ensuring a market share UNITAID, 2016 • • Impact and C/E evaluation Generation of new evidence Scalability Sustainability

POC NAT pipeline Taq. Man® HIV-1 Qual v 2. 0 Roche DBS CE Mark Real. Time Qual HIV-1 Abbott DBS CE Mark (2011) Alere™ q HIV-1/2 Detect Alere CE Mark ZIVA™ Cavidi Under development Ustar Iquum/Roche SAMBA II EID DRW Kenya, Uganda Xpert® HIV-1 Qual Cepheid CE Mark Lumora LYNX HIV p 24 NWGHF Micronics Gene. Xpert Omni Cepheid SAMBA I Semi-Q EID DRW Kenya, Uganda 2014 2015 2016 * Reported November 2015 - timeline and sequence may change no specific market launch date; DBS assay CE Mark noted for laboratory products ** In addition, open polyvalent platforms are currently available for HIV VL and EID testing Source: HIV/AIDS Diagnostics Technology Landscape, UNITAID, 2015 Quantum. D x DFA

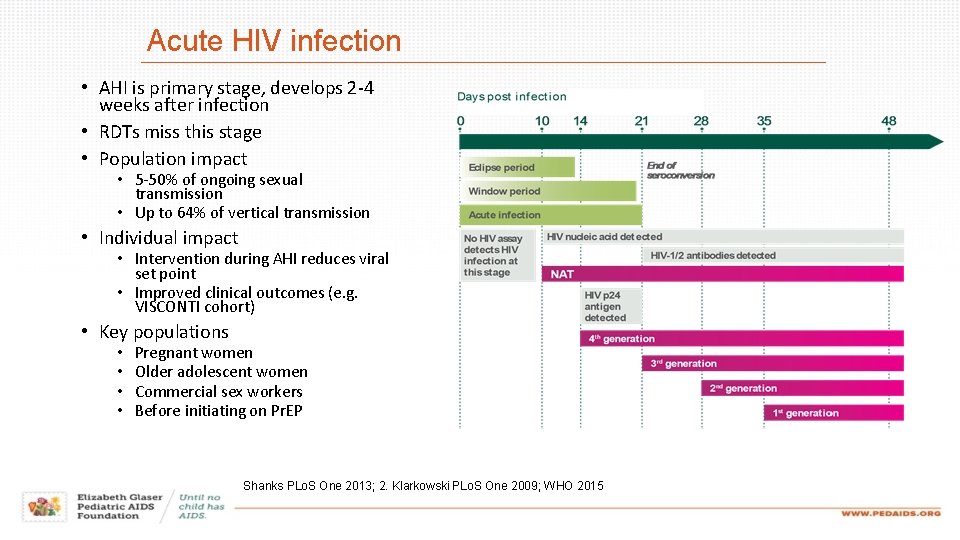
Acute HIV infection • AHI is primary stage, develops 2 -4 weeks after infection • RDTs miss this stage • Population impact • 5 -50% of ongoing sexual transmission • Up to 64% of vertical transmission • Individual impact • Intervention during AHI reduces viral set point • Improved clinical outcomes (e. g. VISCONTI cohort) • Key populations • • Pregnant women Older adolescent women Commercial sex workers Before initiating on Pr. EP Shanks PLo. S One 2013; 2. Klarkowski PLo. S One 2009; WHO 2015

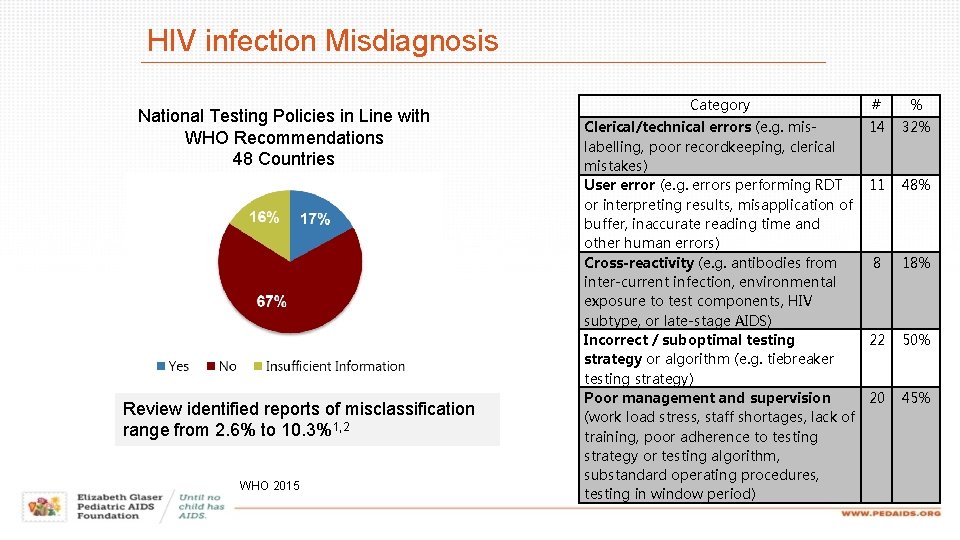
HIV infection Misdiagnosis National Testing Policies in Line with WHO Recommendations 48 Countries Review identified reports of misclassification range from 2. 6% to 10. 3%1, 2 WHO 2015 Category # % Clerical/technical errors (e. g. mislabelling, poor recordkeeping, clerical mistakes) User error (e. g. errors performing RDT or interpreting results, misapplication of buffer, inaccurate reading time and other human errors) Cross-reactivity (e. g. antibodies from inter-current infection, environmental exposure to test components, HIV subtype, or late-stage AIDS) Incorrect / suboptimal testing strategy or algorithm (e. g. tiebreaker testing strategy) Poor management and supervision (work load stress, staff shortages, lack of training, poor adherence to testing strategy or testing algorithm, substandard operating procedures, testing in window period) 14 32% 11 48% 8 18% 22 50% 20 45%

Thank you
 Purpose of nursing process
Purpose of nursing process Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Nursing diagnosis three parts
Nursing diagnosis three parts Nursing process objectives
Nursing process objectives Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis What are tutorials
What are tutorials Proof of concept project management
Proof of concept project management Cobas academy
Cobas academy Vdp ibm
Vdp ibm Poc data management
Poc data management Onet poc
Onet poc Medical automation systems
Medical automation systems