Use of Mesenchymal Stem Cells In Myocardial Infarction








- Slides: 14

Use of Mesenchymal Stem Cells In Myocardial Infarction Treatment 4/5/06 Snehal Patel Advisor: Dr. Bettye Hollins

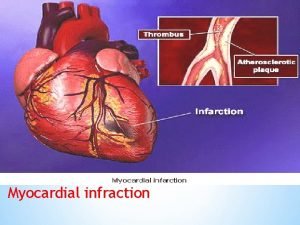
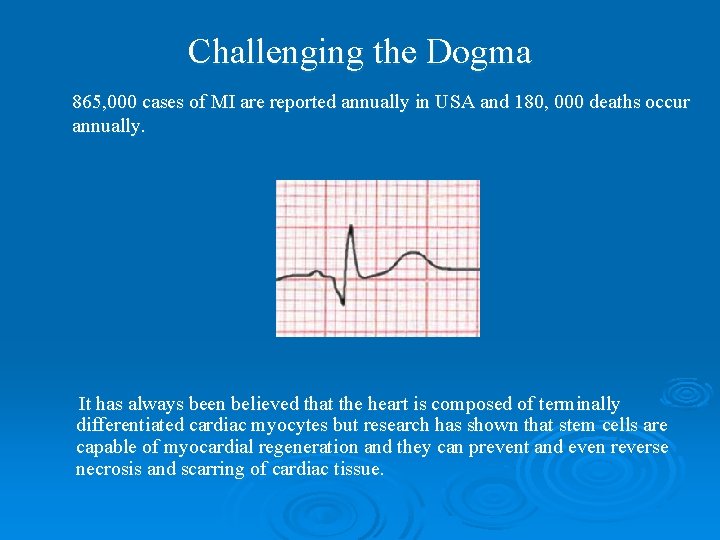
Challenging the Dogma 865, 000 cases of MI are reported annually in USA and 180, 000 deaths occur annually. It has always been believed that the heart is composed of terminally differentiated cardiac myocytes but research has shown that stem cells are capable of myocardial regeneration and they can prevent and even reverse necrosis and scarring of cardiac tissue.

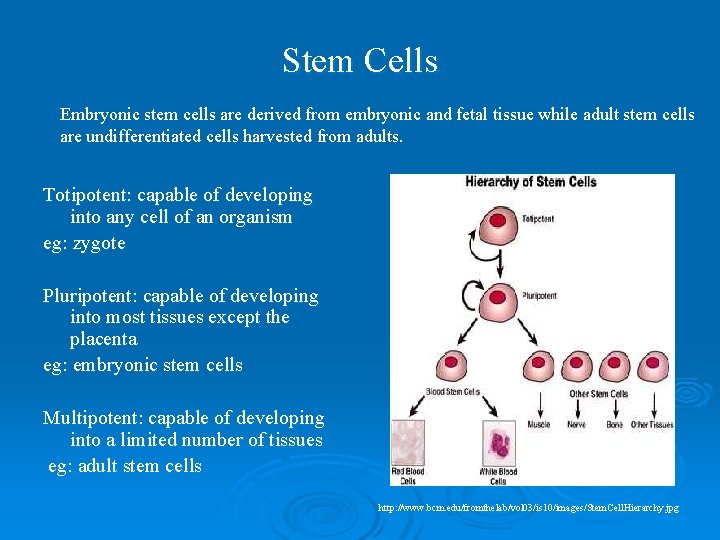
Stem Cells Embryonic stem cells are derived from embryonic and fetal tissue while adult stem cells are undifferentiated cells harvested from adults. Totipotent: capable of developing into any cell of an organism eg: zygote Pluripotent: capable of developing into most tissues except the placenta eg: embryonic stem cells Multipotent: capable of developing into a limited number of tissues eg: adult stem cells http: //www. bcm. edu/fromthelab/vol 03/is 10/images/Stem. Cell. Hierarchy. jpg

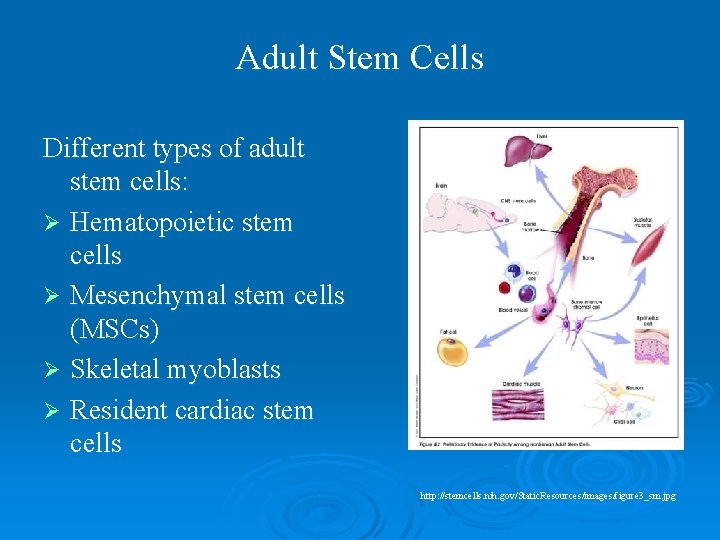
Adult Stem Cells Different types of adult stem cells: Ø Hematopoietic stem cells Ø Mesenchymal stem cells (MSCs) Ø Skeletal myoblasts Ø Resident cardiac stem cells http: //stemcells. nih. gov/Static. Resources/images/figure 3_sm. jpg

Steps in Stem Cell Treatment Ø Extraction of MSCs: They are extracted from the bone marrow of the donor. Ø Expansion of MSCs in culture medium : This is done using off the shelf products like enrichment cocktails and growth mediums. Ø Delivery of the MSCs to the site of the infarct. Ø Homing and differentiation of MSCs: Once in the system the MSCs migrate to the site of the infarct and differentiate into myocytes under the influence of cytokines and paracrine agents.

Routes of Delivery Two main routes of delivery Ø Transvascular: This includes intravenous infusion and intracoronary infusion. Intracoronary infusion is done using percutaneous coronary intervention (PCI). Ø Direct injection into the myocardium: In this approach the MSCs are directly injected into the myocardium at the borders of the site of the infarct (endocardial) using a needle catheter during a PCI or (intramyocardial) as an adjunct to a coronary bypass graft (CABG)

Routes of Delivery Intravenous § Least invasive § More effective in acute settings § More systemic exposure Intracoronary § Intermediate § More effective in acute settings § Less systemic exposure § High rate of engraftement at the site of infarct Direct Myocardial injection § Most invasive § Can be used later as compared to intravascular approaches § Least systemic exposure § High rate of engraftment at the rite of infarct

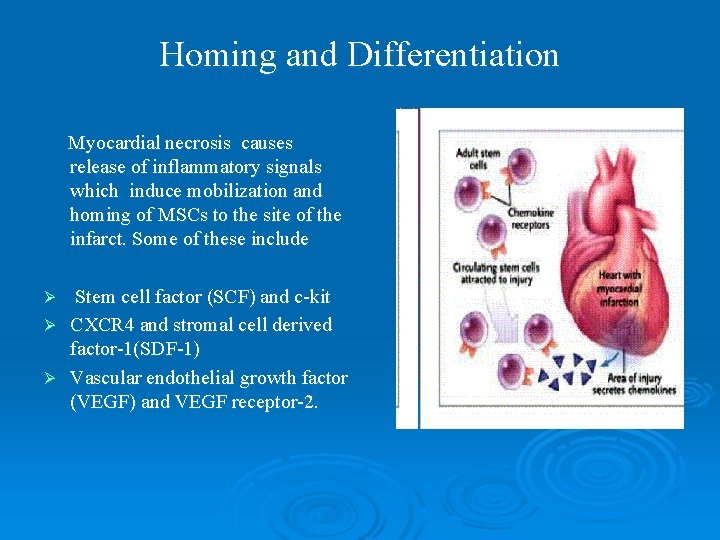
Homing and Differentiation Myocardial necrosis causes release of inflammatory signals which induce mobilization and homing of MSCs to the site of the infarct. Some of these include Stem cell factor (SCF) and c-kit Ø CXCR 4 and stromal cell derived factor-1(SDF-1) Ø Vascular endothelial growth factor (VEGF) and VEGF receptor-2. Ø

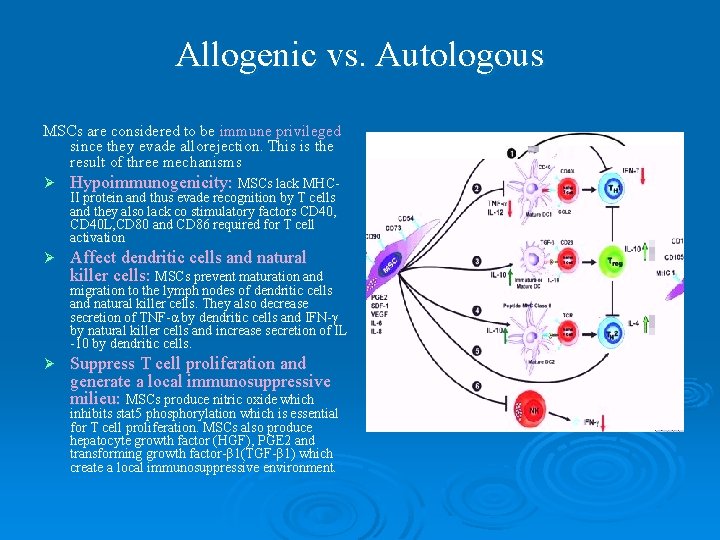
Allogenic vs. Autologous MSCs are considered to be immune privileged since they evade allorejection. This is the result of three mechanisms Ø Hypoimmunogenicity: MSCs lack MHC- Ø Affect dendritic cells and natural killer cells: MSCs prevent maturation and II protein and thus evade recognition by T cells and they also lack co stimulatory factors CD 40, CD 40 L, CD 80 and CD 86 required for T cell activation migration to the lymph nodes of dendritic cells and natural killer cells. They also decrease secretion of TNF-α by dendritic cells and IFN-γ by natural killer cells and increase secretion of IL -10 by dendritic cells. Ø Suppress T cell proliferation and generate a local immunosuppressive milieu: MSCs produce nitric oxide which inhibits stat 5 phosphorylation which is essential for T cell proliferation. MSCs also produce hepatocyte growth factor (HGF), PGE 2 and transforming growth factor-β 1(TGF-β 1) which create a local immunosuppressive environment.

Conduction After the MSCs undergo differentiation it is essential that they also acquire the electrical properties of cardiac myocytes. Electrical conduction through the differentiated MSCs is attributed to the development of gap junctions, which are seen at the interfaces between the MSCs themselves and between the MSCs and cardiac myocytes. MSCs have a resting potential of -30 to -40 m. V. They express a small fraction of Ltype Ca channels and they are considered inexcitable which results in slower conduction velocity in-vitro, in a co-culture of MSCs and myocytes as compared to only myocytes. But the conduction velocity in a co-culture of MSCs and myocytes is still faster than that observed in a co-culture of fibroblasts and myocytes, which would be seen in the case of an MI. Resynchronization of two separately beating fields has been seen within 24 to 48 hours of transplantation.

Risks vs. Benefits: Clinical trials have shown improvement of both systolic and diastolic function after transplant of MSC Ø Ø Ø Increase in left ventricular ejection fraction (LVEF) Decrease in the area of functional defect Increase in the wall movement velocity of the infarcted area No significant changes in the left ventricular dystolic diameter (LVDd) No significant changes in E/A ratio Small increase in isovolumic relaxation time (IVRT) Risks: Ø Ø Highly invasive procedures except for IV infusion Susceptible to re-entrant arrhythmias Risk of propagating genetic defects Tumors

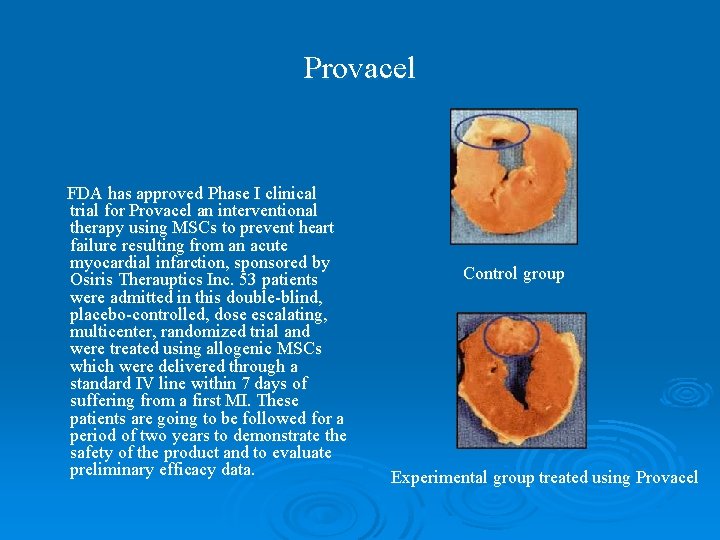
Provacel FDA has approved Phase I clinical trial for Provacel an interventional therapy using MSCs to prevent heart failure resulting from an acute myocardial infarction, sponsored by Osiris Therauptics Inc. 53 patients were admitted in this double-blind, placebo-controlled, dose escalating, multicenter, randomized trial and were treated using allogenic MSCs which were delivered through a standard IV line within 7 days of suffering from a first MI. These patients are going to be followed for a period of two years to demonstrate the safety of the product and to evaluate preliminary efficacy data. Control group Experimental group treated using Provacel

Conclusion Preclinical and human trials have showed short term benefits of using MSCs post MI that include improvement in LVEF, reduction of scar tissue, absence of hypertrophy and improvement in in contractility and conduction but it is also essential to study long term effects. Further studies are warranted to help design treatments that are best suited to individual needs depending on the location of the infarct, time elapsed since the MI and hemodynamic stability of the patient. Thus although MSC transplants have a long way to go from the laboratory to the patient’s bedside, they do show promise, that their use will help improve the quality of life of the patients and reduce progression to heart failure.

References Ø Ø Ø Ø Ø Aggarwal S and Pittenger MF. Human Mesenchymal stem cells modulate allogenic immune cell responses. Blood. 2005; 105(4): 1815 -1822. Beeres S, Atsma DE, van der Larse A, Pijnappels DA, van Tuyn J, Fibbe WE et al. Human adult bone marrow stem cells repair experimental conduction block in rat cardiomyocytes cultures. Journal of American Collegd of Cardiology. 2005; 46(10): 1943 -1952. Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P et al. Proarrhythmis potential of mesenchymal stem cell transplantation revealed in an invitro coculture model. Circulation. 2006; 113: 1832 -1841. Chen S, Fang W, Ye F, Liu Y, Qian J, Shan S et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. American Journal of Cardiology. 2004; 94: 92 -95. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99(10): 3838 -3843. Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, et al. A quantitative randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. European Heart Journal. 2006; 27(9): 1114 -1122. Ge J, Li Y, Qian J, Shi J, Wang Q, Niu Y et al. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction(TCT-STAMI). Heart. 2006; 92: 1764 -1767. Hou M, Yang K, Zhang H, Zhu W, Duan F, Wang H, et al. Transplantation of mesenchymal stem cells from human bone marrow improves damaged heart function in rats. International Journal of Cardiology. In press 2006; Orlic D, Hill J M, Arai A E. Stem cells for myocardial regeneration. Circulation Research. 2002; 91: 1092 -1102. Osiris Therapeutics Inc. Provacel clinical trial infnrmation. Available at http: //www. osiristx. com/clinical_trials_provacel. php. . Accessed Feb 19, 2007. Poh K, Sperry E, Young RG, Freyman T, Barringhaus KG, Thompson CA. Repeated direct endomyocardial transplantation of allogenic stem cells: Safety of “off-the-shelf” cellular cardiomyoplasty strategy. International Journal of Cardiology. In press 2006; Reffelmann T and Kloner RA. The “no-reflow” phenomenon: basic science and correlates. Heart. 2002; 87: 162 -168. Ryan JM, Barry FP, Murphy JM and Mahon BP. Mesenchylal stem cells avoid allogenic rejection. Journal of Inflammation. 2005; 2(8): Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays critical role in suppression of T cell proliferation my mesenchymal stem cells. Blood. 2007; 109(1): 228 -234. Schaefer A, Meyer GP, Fuchs M, Klein G, Kaplan M, Wollert K, et al. Impact of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: results from BOOST trial. European Heart Journal. 2006; 27: 929 -35. Thorn T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Monalio T, et al. Heart disease and Stroke statidtics-2006 update: A report from the American Heart Association statistics committee and Stroke statistics subcommittee. Circulation. 2006; 113: 85 -151. Tse WT, Pendelton JD, Beyer WM, Egalka MC And Guinan EC. Supression of allogenic T cell proliferation by human stromal marrow cells: implications in transplantation. Transplantation. 2003; 75(3): 389 -387. Valiunas V, Doronin S, Valiuniene L, Potatpova I, Zuckerman J, Walcott B, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. Journal of Physiology. 2004; 555(3): 617 -626. Weissberg PL, Qasim Asif. Stem cell therapy for myocardial repair. Heart. 2005; 91: 696 -702. Wolldrt KC, Drexler H. Clinical Applications of Stem Cells for the Heart. Circulation Research. 2005; 96: 151 -162.
 Pico question myocardial infarction
Pico question myocardial infarction Myocardinal infraction
Myocardinal infraction Anterior leads
Anterior leads Pancreas wiki
Pancreas wiki Acute pericarditis
Acute pericarditis Undifferentiated mesenchymal cells
Undifferentiated mesenchymal cells Undifferentiated mesenchymal cells
Undifferentiated mesenchymal cells Outline the cell theory
Outline the cell theory Epithelial mesenchymal
Epithelial mesenchymal Types of metaplasia
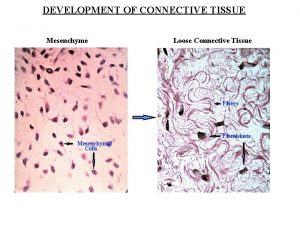
Types of metaplasia Development of connective tissue
Development of connective tissue Gross
Gross Subendocardial infarction
Subendocardial infarction Patient synonm
Patient synonm Myocardial ischemia meaning
Myocardial ischemia meaning