Units of Measurement All measurements must include the



















- Slides: 20

Units of Measurement • All measurements must include the number and the unit • Ex: 4. 5 m or 23 g/m. L • Use SI System- International System of Units which includes the metric system

SI Units • • • Length= meter (m) Mass= kilogram (kg) Time= seconds (s) Temperature= Kelvin (K) *** Pressure= pascal (Pa)

Derivied units • Area= m 2 or cm 2 • Volume= cm 3 • Density= g/cm 3 or g/m. L

Non SI Units we will use • Volume= Liter (L) or mililiter m. L • Temperature= Celsius (o. C) • Pressure= atmosphere (atm) or millimeters of mercury (mm. Hg)

Units we NEVER measure in !!! • • • Fahrenheit °F Inches, yards, feet etc. Ounces, pounds Cups, teaspoons, etc. PSI (pounds per square inch)

Metric Prefixes • • • Tera- Trillion (T) ex: Giga- Billion (G) ex: Mega- Million (M) ex: Kilo- Thousand (k) ex: Hecto- Hundred (h) ex: Deca- Ten (da) ex: Deci- tenth (d) ex: Centi- hundredth (c) ex: Milli- thousandth (m) ex: Micro- millionth (µ) ex: Nano- billionth (n) ex: Pico-trillionth (p) ex:

Uncertainty in Measurements • When you take down a measurement, take down all the numbers an instrument gives you PLUS one uncertain number you estimate • Ex: 135. 16 grams, the measurement is accurate only to 135. 1 The last digit is not accurate • If you get an exact amount put a decimal behind the last digit. • Ex: 150. g • Yes you can have hanging decimals & if your math teacher says other wise send them to see me

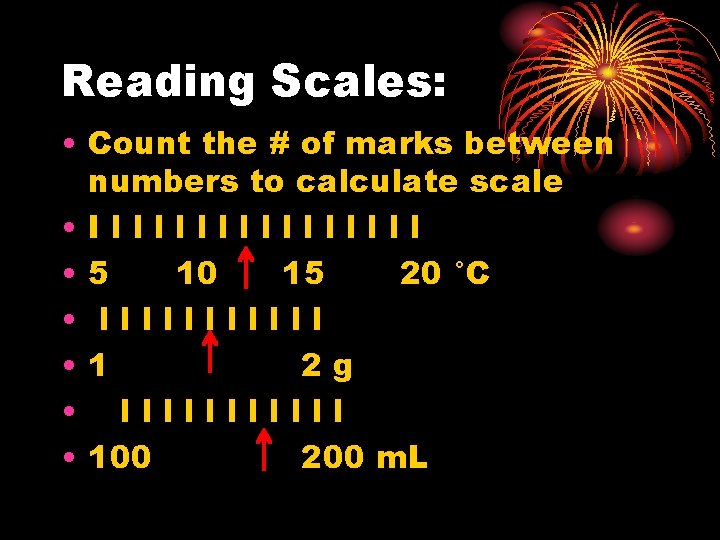
Reading Scales: • Count the # of marks between numbers to calculate scale • Illllllll • 5 10 15 20 °C • llllll • 1 2 g • llllll • 100 200 m. L

Why are measurements uncertain? • Human Error • Instruments are not flawless. • Always involves some estimation • Digital- the last digit is estimated. • Scales- human estimation

Significant Figures and Scientific Notation

Rules for Significant Figures (aka sig figs) • If your # > 1 (w/o a decimal) then. Count all whole # and zero’s inbetween Ex: 1234= 105= 300= 2030=

Sig Fig Rules continued • If your # is < 1 then • Count the first whole # and all the numbers after it (including zero’s) • Ex: 0. 00234 = 0. 2010 = 0. 000003 = 0. 00300000 =

Last Sig Fig Rule • If the #>1 with a decimal then • Count ALL the #s • Ex: 2. 340 = • 500. 55 = • 100. 000 =

Adding and Subtracting • Use the least number of decimal places 5. 000 cm +4. 352 cm 9. 352 cm 5. 000 cm +4. 3 cm 9. 3 cm Your only as “strong” as the weakest” link

• 5. 00 cm - 4. 352 cm 2 decimal places 3 decimal places 0. 648 cm • 0. 65 cm is the correct answer in sig figs

• • • 430 mm * This one is tricky! + 23 mm 453 mm Still have to use the least significant place holder 430 is only significant to the ten’s place so the correct answer (in sig figs) is 450 mm, not 453 mm Why? an answer can only be as precise as the least precise number used in the calculation

Multiplying and Dividing • multiply or divide the numbers • count the total number of sig figs in each number • your final answer should have no more sig figs than the lowest number of sig figs you started with

• 6. 7 cm x 1. 1 cm • 7. 37 cm 2 • • 7. 4 cm 2 is the correct answer in sig figs

Scientific Notation • In chemistry we often use very large or very small numbers. • 54, 000 • 0. 000008765 • We want to be able to show these numbers without all insignificant zeros, so we use scientific notation.

• 1. Make the number less than ten • 2. Count the number of places the decimal moves = the exponent. • 3. If the original number is less than one the exponent is negative, if greater than one its positive • Ex: 54 000 = • Ex: 0. 000008765=
 #include stdio.h #include stdlib.h #include string.h
#include stdio.h #include stdlib.h #include string.h #include stdio.h #include conio.h #include stdlib.h
#include stdio.h #include conio.h #include stdlib.h Unit of quantity
Unit of quantity English linear measurements
English linear measurements Is string included in iostream
Is string included in iostream Int main()
Int main() #include iostream #include string using namespace std
#include iostream #include string using namespace std #include iostream #include cmath
#include iostream #include cmath H
H #include iostream que es
#include iostream que es Cppinclude
Cppinclude Namespace string
Namespace string Mr. gallon
Mr. gallon Unit for momentum
Unit for momentum The customary system
The customary system