UNIT SEVEN Earths Water Chapter 21 Water and























- Slides: 26


UNIT SEVEN: Earth’s Water § Chapter 21 Water and Solutions § Chapter 22 Water Systems § Chapter 23 How Water Shapes the Land


Chapter Twenty-One: Water and Solutions § 21. 1 Water § 21. 2 Solutions § 21. 3 Acids, Bases, and p. H

Chapter 21. 3 Learning Goals § Differentiate between acids and bases. § Define p. H. § Explain the significance of acids, bases, and p. H to living organisms and the environment.

21. 3 What are acids? § An acid is a compound that dissolves in water to make a particular kind of solution. § Chemically, an acid is any substance that produces hydronium ions (H 3 O+) when dissolved in water.

21. 3 What are acids? Some properties of acids are: 1. Acids create the sour taste in food, like lemons. 2. Acids react with metals to produce hydrogen (H 2) gas. 3. Acids change the color of blue litmus paper to red. 4. Acids can be very corrosive, destroying metals and burning skin through chemical action.

21. 3 Bases § A base is any substance that dissolves in water and produces hydroxide ions (OH-).

21. 3 What are bases? Some properties of bases are: 1. Bases create a bitter taste. 2. Bases have a slippery feel, like soap. 3. Bases change the color of red litmus paper to blue. 4. Bases can be very corrosive, destroying metals and burning skin through chemical action.



21. 3 Acids and bases § One of the most important properties of water is its ability to act as both a weak acid or as a weak base. § In the presence of an acid, water acts as a base. § In the presence of a base, water acts as an acid.


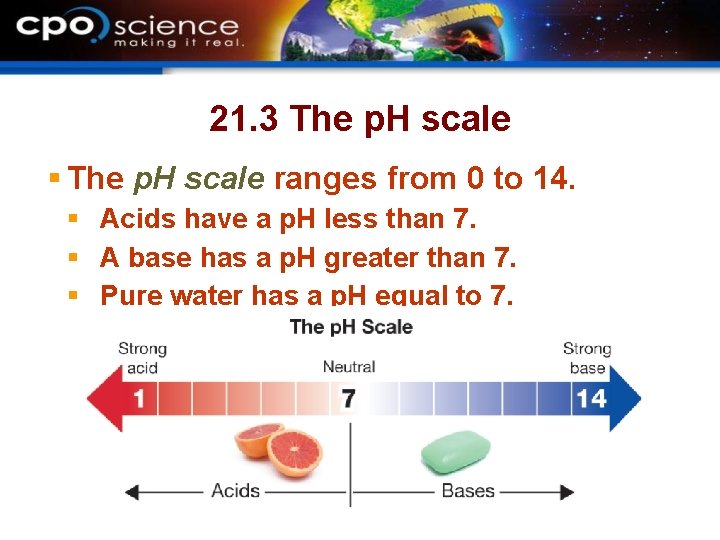
21. 3 The p. H scale § The p. H scale ranges from 0 to 14. § Acids have a p. H less than 7. § A base has a p. H greater than 7. § Pure water has a p. H equal to 7.

21. 3 The p. H scale § Red and blue litmus paper are p. H indicators that test for acids or bases.


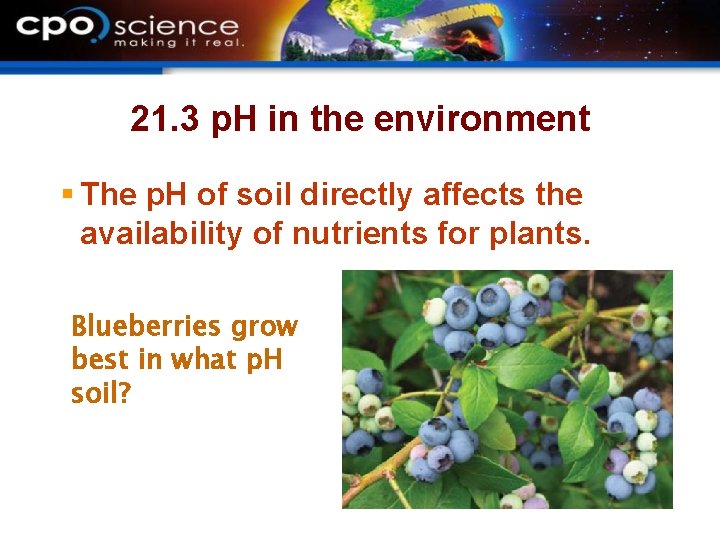
21. 3 p. H in the environment § The p. H of soil directly affects the availability of nutrients for plants. Blueberries grow best in what p. H soil?

21. 3 p. H in the environment § The p. H of water directly affects aquatic life. How are frogs and amphibians sensitive to p. H changes?


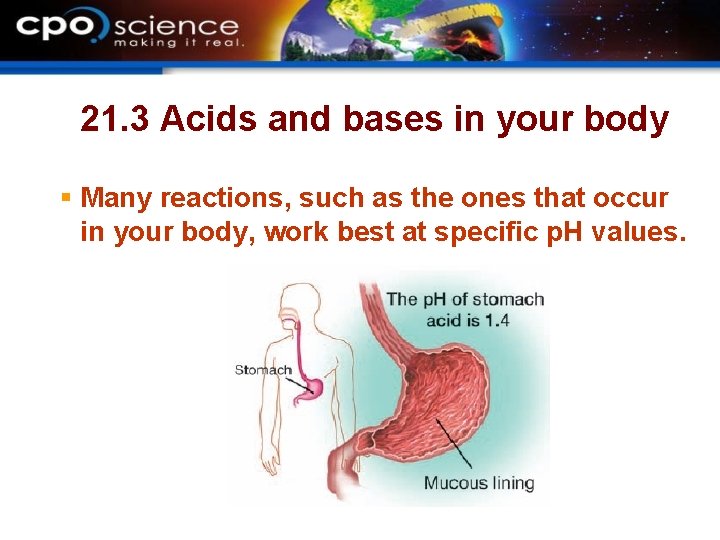
21. 3 Acids and bases in your body § Many reactions, such as the ones that occur in your body, work best at specific p. H values.

21. 3 p. H and blood § The p. H of your blood is normally within the range of 7. 3– 7. 5. § Holding your breath causes blood p. H to drop. § High blood p. H can be caused by hyperventilating.

21. 3 Neutralization reactions § When acid and base solutions are mixed in the right proportions, the positive ions from the base combine with the negative ions from the acid. § A new ionic compound forms and water is one of the products. § This is a neutralization reaction.


21. 3 Neutralization reaction § Neutralization goes on in your body every day as food and digestive fluids leave the stomach. § Having soil that is too acidic is a common problem in the U. S. for farmers and gardeners.

Investigation 21 C Acids, Bases, and p. H § Key Question: What is p. H?

Are You Feeling a Little Sour? § By nature, our slightly alkaline p. H needs to remain balanced. Yet what we eat and drink changes our p. H. § For example, if you eat a lot of meat and no vegetables, your p. H becomes acidic.
 Water and water and water water
Water and water and water water Seven deadly sins seven heavenly virtues
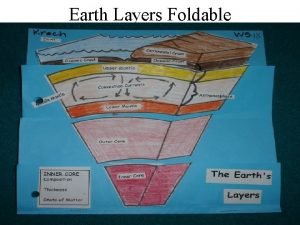
Seven deadly sins seven heavenly virtues Earth layers foldable
Earth layers foldable Earths roation
Earths roation Whats earths moon called
Whats earths moon called Home sweet biome crossword
Home sweet biome crossword Most abundant element in earth's crust
Most abundant element in earth's crust Basalt
Basalt Whats earths moon called
Whats earths moon called Which layer of the earth slowly moves like putty
Which layer of the earth slowly moves like putty Earths early atmosphere contained
Earths early atmosphere contained Earth layer foldable
Earth layer foldable Earths major crustal plates
Earths major crustal plates Earths orbit seasons
Earths orbit seasons Brown earth soil profile
Brown earth soil profile Study of earth's physical features
Study of earth's physical features Earth's honey
Earth's honey What is luna moon
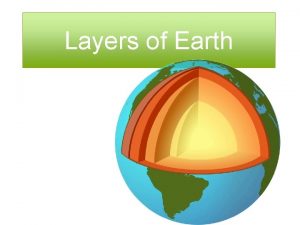
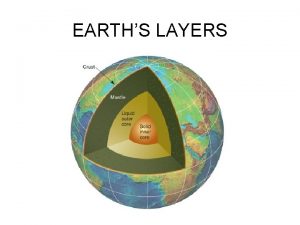
What is luna moon Earths mantle
Earths mantle Earths crust
Earths crust Earths interior
Earths interior Spring earth tilt
Spring earth tilt Atmospheric definition
Atmospheric definition What is the true shape of earth
What is the true shape of earth Arch of constantine dimensions
Arch of constantine dimensions What does earths tilt do
What does earths tilt do Earths boundaries
Earths boundaries