Unit D Topic 1 STATIC ELECTRICITY Static Electricity










- Slides: 28

Unit D Topic 1

STATIC ELECTRICITY • • Static Electricity is a stationary electrical charge. Some examples of what it can be caused by are: • Lightening • Shocks from touching doorknobs • Clothes clinging together It is produced from the rubbing or touching objects together

LET’S TRY IT OUT. . . Experiment #1 • Blow up a balloon • Rub the balloon on your clothing • Hold the balloon by your hair • What happens? Experiment #2 • Rub the balloon on your clothing again • Get with a partner • Hold your balloons by the knot • Bring the rubbed surface of your balloons slowly together • What happens?

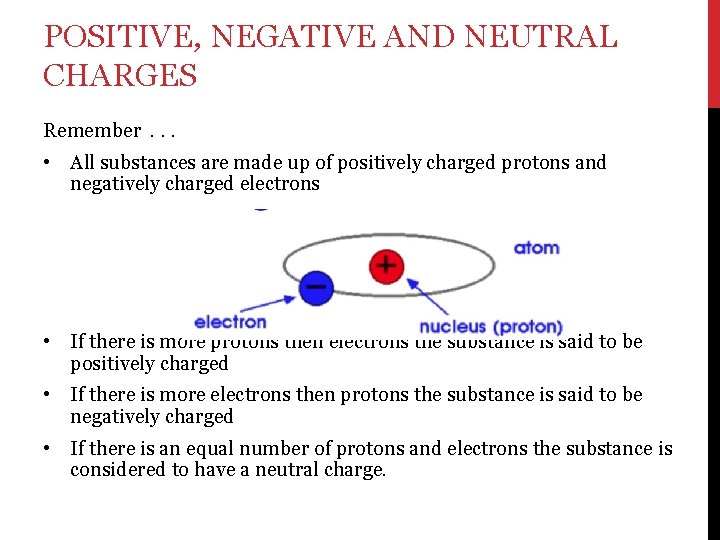
POSITIVE, NEGATIVE AND NEUTRAL CHARGES Remember. . . • All substances are made up of positively charged protons and negatively charged electrons • If there is more protons then electrons the substance is said to be positively charged • If there is more electrons then protons the substance is said to be negatively charged • If there is an equal number of protons and electrons the substance is considered to have a neutral charge.

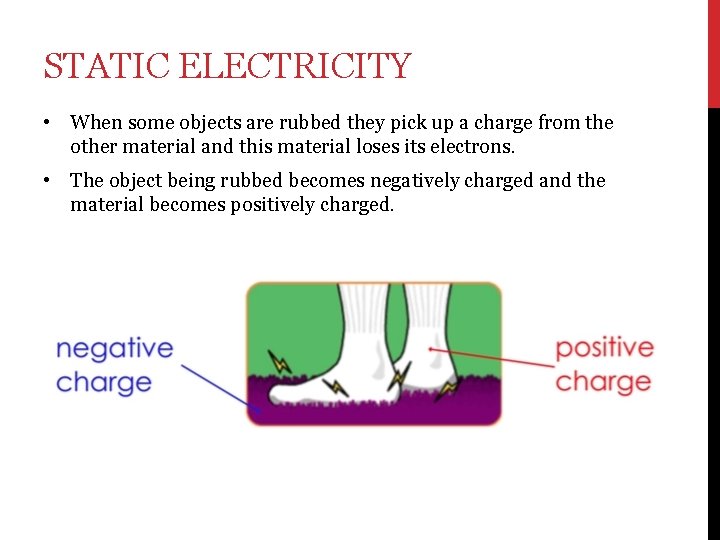
STATIC ELECTRICITY • When some objects are rubbed they pick up a charge from the other material and this material loses its electrons. • The object being rubbed becomes negatively charged and the material becomes positively charged.

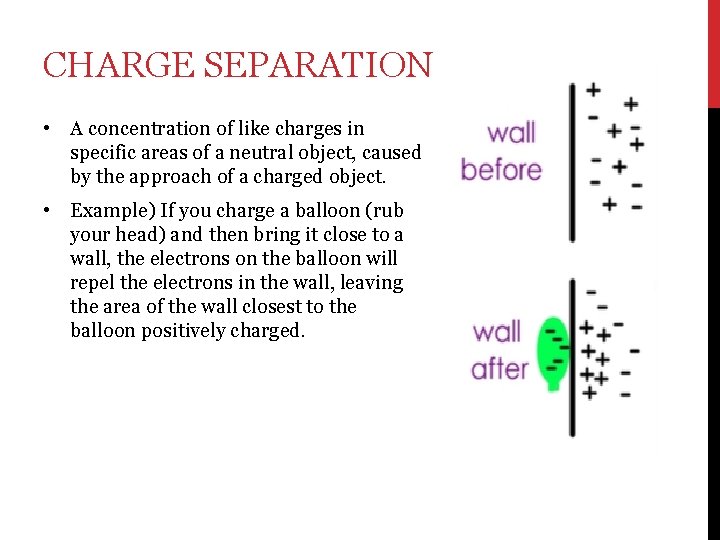
CHARGE SEPARATION • A concentration of like charges in specific areas of a neutral object, caused by the approach of a charged object. • Example) If you charge a balloon (rub your head) and then bring it close to a wall, the electrons on the balloon will repel the electrons in the wall, leaving the area of the wall closest to the balloon positively charged.

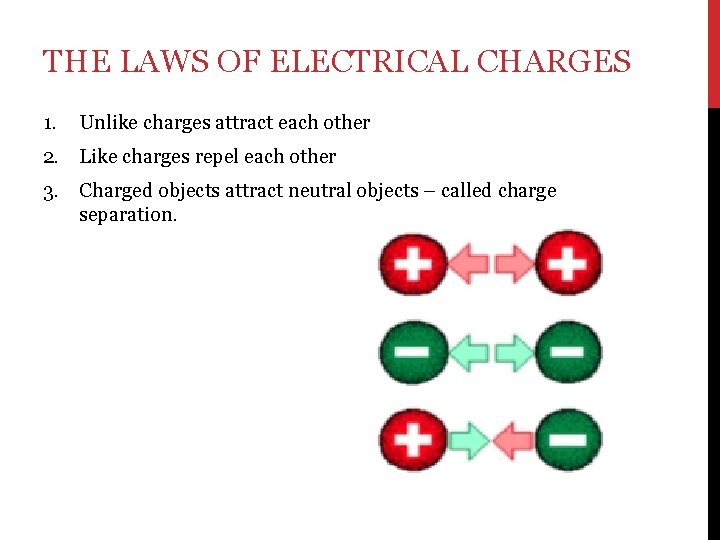
THE LAWS OF ELECTRICAL CHARGES 1. Unlike charges attract each other 2. Like charges repel each other 3. Charged objects attract neutral objects – called charge separation.

NEUTRALIZING CHARGES • • • Electrical discharge: sudden transfer of electrical charge from one object to another. • Indicated by a shock (can usually feel or see this) Neutralized: the removal of charge (through electrical discharge) so that the overall charge is neutral Grounding; connecting an object to ground with a conducting wire. This is an easy way to neutralize a conducting material.

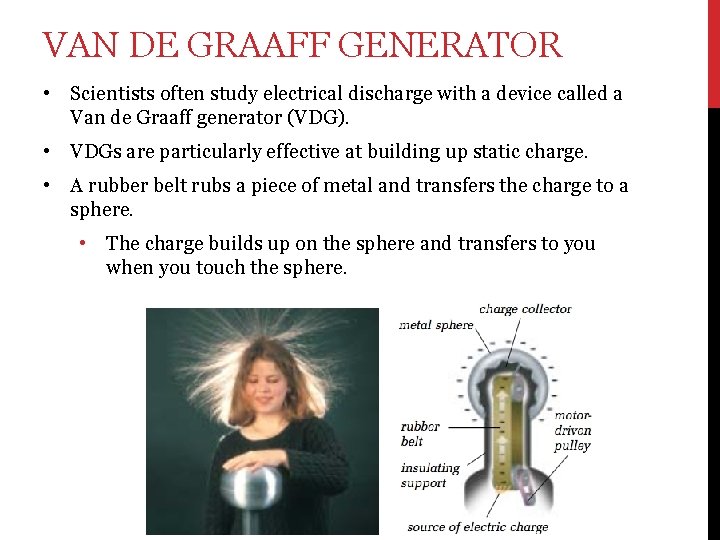
VAN DE GRAAFF GENERATOR • Scientists often study electrical discharge with a device called a Van de Graaff generator (VDG). • VDGs are particularly effective at building up static charge. • A rubber belt rubs a piece of metal and transfers the charge to a sphere. • The charge builds up on the sphere and transfers to you when you touch the sphere.

ELECTRICAL CURRENT • Electrical current – the steady flow of charged particles This is the type of electricity needed to operate electrical devices Electrical current flows continuously as long as two conditions are met: • • 1. The flow of electrical current requires an energy source 2. Electrical current will not flow unless it has a complete path or circuit for the charged particles to flow through


AMPERES • The rate at which an electrical current flows is measured in amperes (A) • Often called “amp” for short, the ampere is named in honour of Andre-Marie Ampere

CONDUCTORS • A key problem is how to move the charge from where it is produced to where it is needed • There are many materials that electrical charge can move through easily – these are known as conductors • Conduction of electricity through wires allows for the transfer of electrical energy from place to place

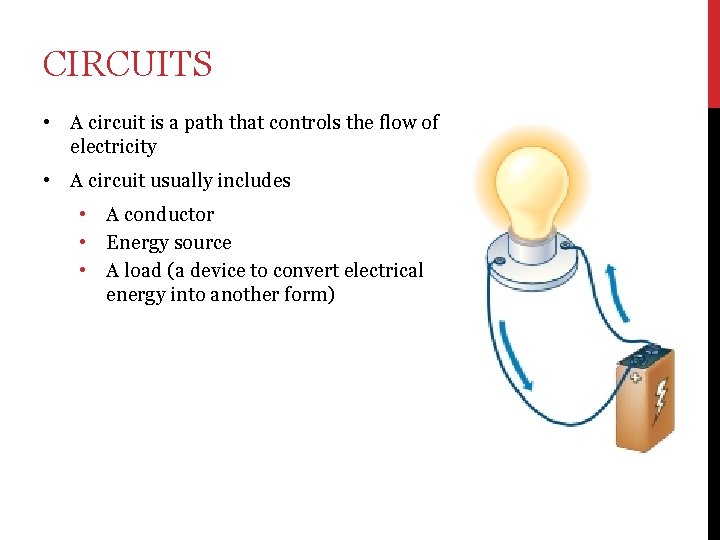
CIRCUITS • A circuit is a path that controls the flow of electricity • A circuit usually includes • • • A conductor Energy source A load (a device to convert electrical energy into another form)

ELECTRICAL ENERGY AND VOLTAGE • Electrical energy is the energy carried by charged particles • Voltage is the measure of how much electrical energy each charged particle carries The higher the voltage the greater the potential energy of each particle • Aka Potential Difference Voltage is measured in volts (V) after Alessandro Volta • •

• Measuring Voltage • • • Voltmeter – an instrument for measuring potential difference in volts The red lead attaches to the positive terminal The black lead to the negative terminal

ELECTRICAL SAFETY Using your textbook pages 284 - 287 to answer the following questions.

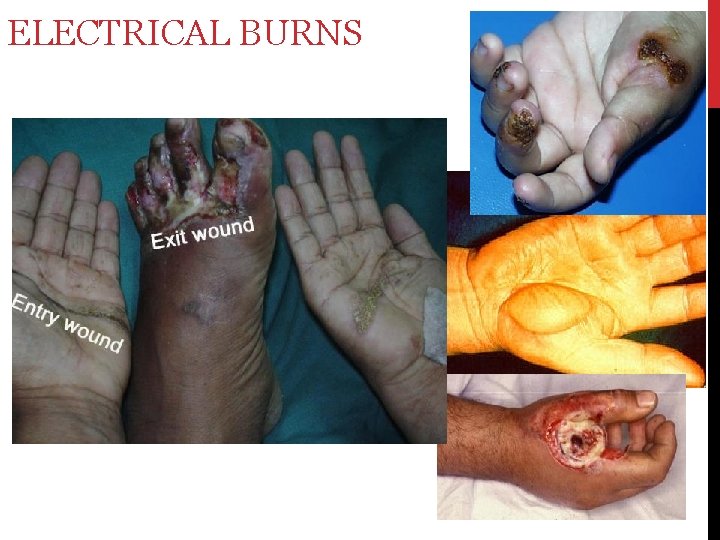
ELECTRICAL BURNS


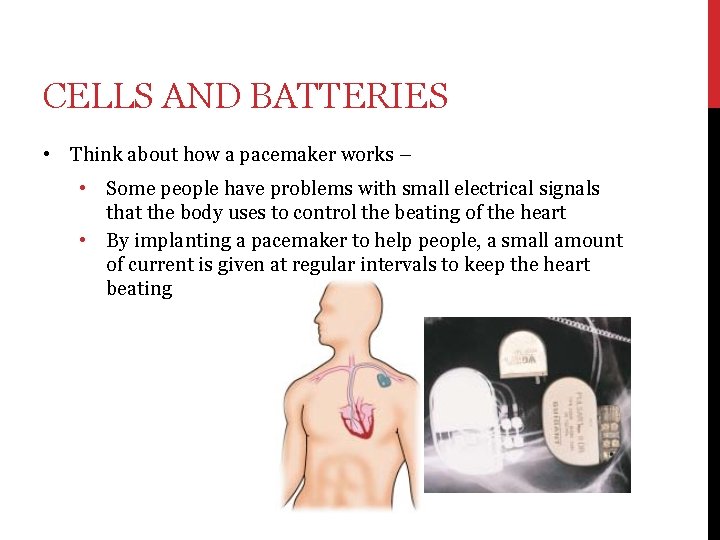
CELLS AND BATTERIES • Think about how a pacemaker works – • • Some people have problems with small electrical signals that the body uses to control the beating of the heart By implanting a pacemaker to help people, a small amount of current is given at regular intervals to keep the heart beating

ELECTROCHEMICAL CELLS • The electricity used to operate a pacemaker comes from an electrochemical cell that supplies a steady current. Is a package of chemicals designed to produce small amounts of electricity • The electricity of the cell comes from chemical reactions A battery is when more than one cell are joined together. • •

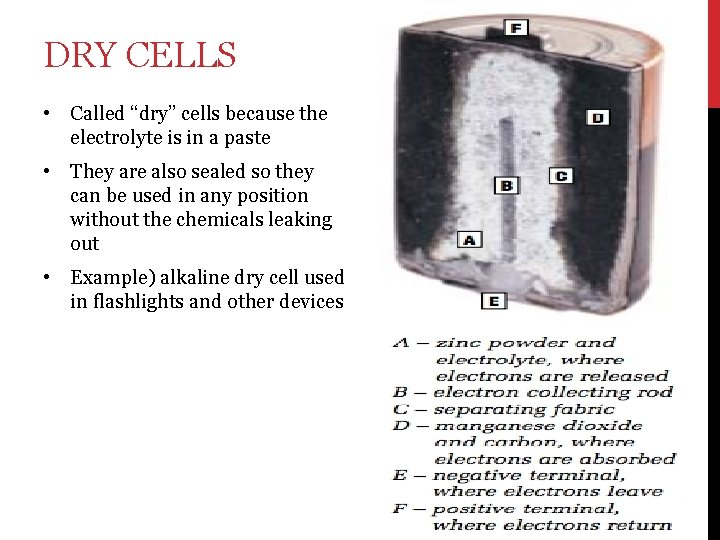
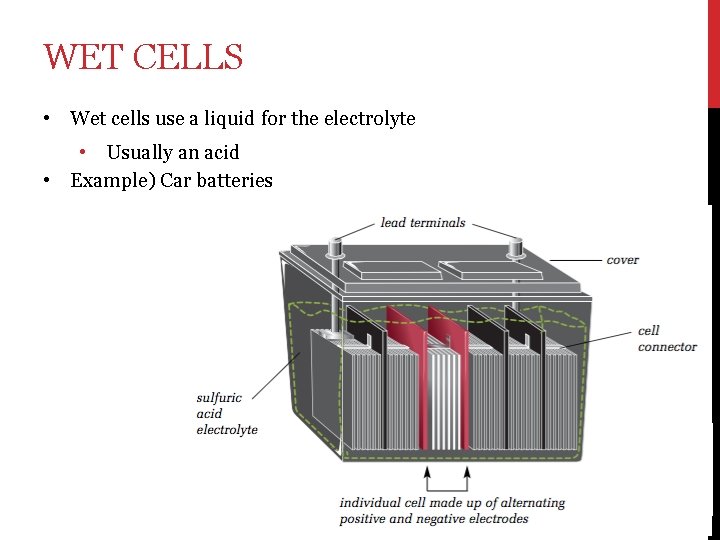
• 1. Cells have three main parts: An electrolyte – a paste or liquid that can form ions and conduct electricity 2. Positive electrode 3. Negative electrode • The electrons flow from the negative electrode → device → positive electrode

DRY CELLS • Called “dry” cells because the electrolyte is in a paste • They are also sealed so they can be used in any position without the chemicals leaking out • Example) alkaline dry cell used in flashlights and other devices

WET CELLS • Wet cells use a liquid for the electrolyte • • Usually an acid Example) Car batteries


RECHARGEABLE CELLS • Also known as secondary cells • Rechargeable cells use different chemicals so that the chemical reaction can be reversed. • An external electrical source is required to reverse the flow of electrons and thus re-establish the original chemicals. • Example) Cell phones (Ni. MH or Ni. Cd) • However, original chemicals aren’t re-formed perfectly each time so even rechargeable batteries wear out

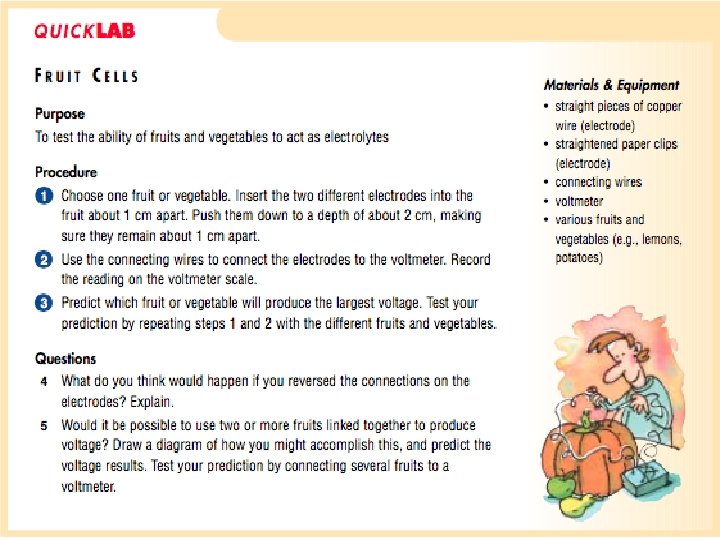
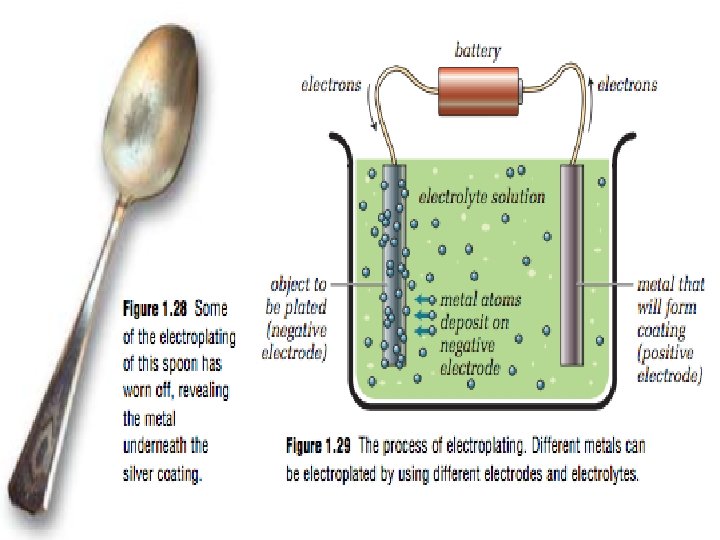
ELECTROCHEMISTRY • Through experimentation, scientists discovered that electrodes and electrolytes can help accomplish a number of things: 1. Electrolysis – uses electrical currents to separate elements from solution Example) chlorine (Cl) can be separated to produce Polyvinyl chloride (PVC) Electroplating – uses electrical currents to transfer metal atoms from one type of electrode to another • 2. 3. 4. • Example) gold plating Anodizing – uses electrical currents to make certain types of metals stronger by adding metal oxides • Example) Anodized aluminum Electrorefining – uses electrical currents to purify certain metals • Purifying gold
