Static Electricity What is Static Electricity n n



- Slides: 11

Static Electricity

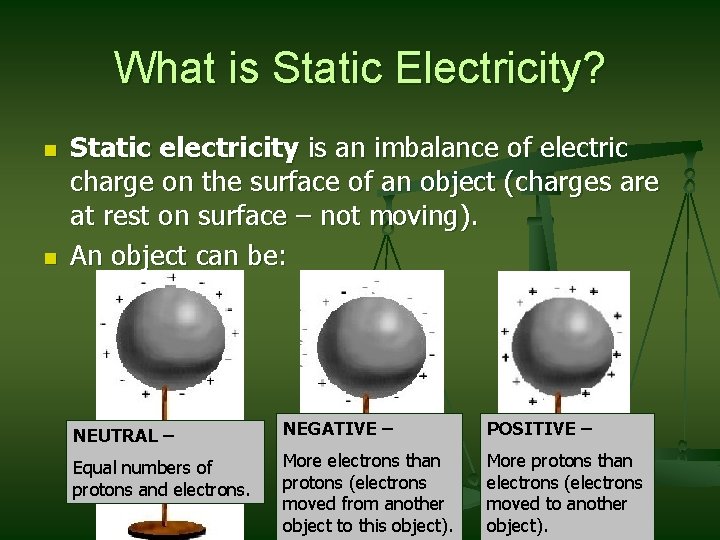
What is Static Electricity? n n Static electricity is an imbalance of electric charge on the surface of an object (charges are at rest on surface – not moving). An object can be: NEUTRAL – NEGATIVE – POSITIVE – Equal numbers of protons and electrons. More electrons than protons (electrons moved from another object to this object). More protons than electrons (electrons moved to another object).

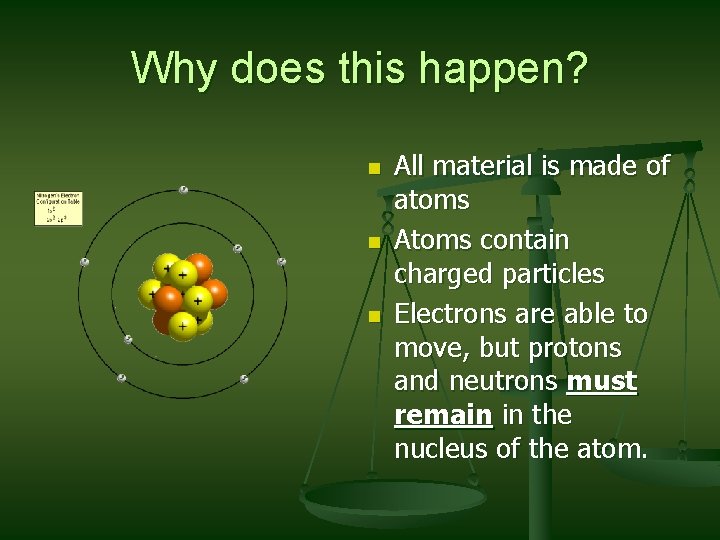
Why does this happen? n n n All material is made of atoms Atoms contain charged particles Electrons are able to move, but protons and neutrons must remain in the nucleus of the atom.

Law of Electric Charges 1. 2. Objects that have the same charge repel each other. Objects that have opposite charge attract each other. What happens when a charged object is brought toward a neutral object?

Induced Charge Separation n If a charged (positive or negative) object is brought near a neutral object, the charged object causes (induces) the electrons to shift. Examples (drawn in notes) – pith ball, metal leaf electroscope. Homework: Pg. 471 #2 -8 (if not done yet)

Why is it called STATIC electricity? n n Static = not moving, staying in one place The charge stays around the area where rubbing occurs. It does NOT travel to the far end of the material.

Charging objects by contact There are 2 different ways to spread charge: 1. Charge by friction – materials are charged when they are rubbed together. - Some atoms attract electrons more than others. Electrons can move from one object to another through friction. - Why does it occur more often in winter? Examples – Rubbing feet on carpet, or comb through hair.

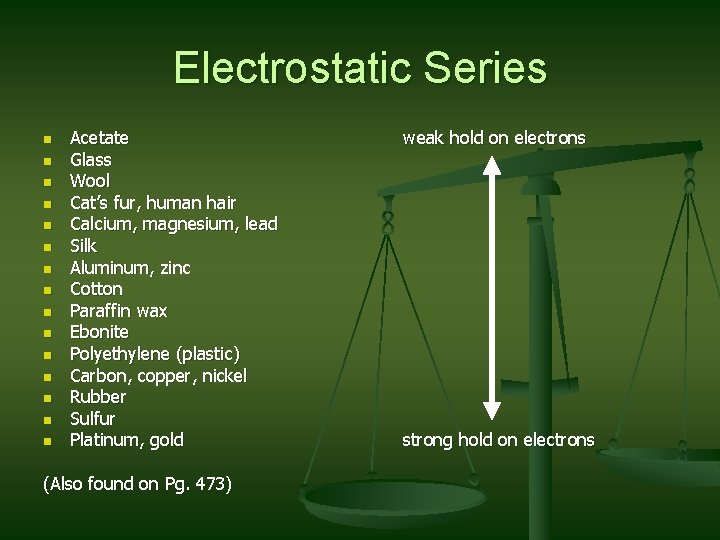
Electrostatic Series n n n n Acetate Glass Wool Cat’s fur, human hair Calcium, magnesium, lead Silk Aluminum, zinc Cotton Paraffin wax Ebonite Polyethylene (plastic) Carbon, copper, nickel Rubber Sulfur Platinum, gold (Also found on Pg. 473) weak hold on electrons strong hold on electrons

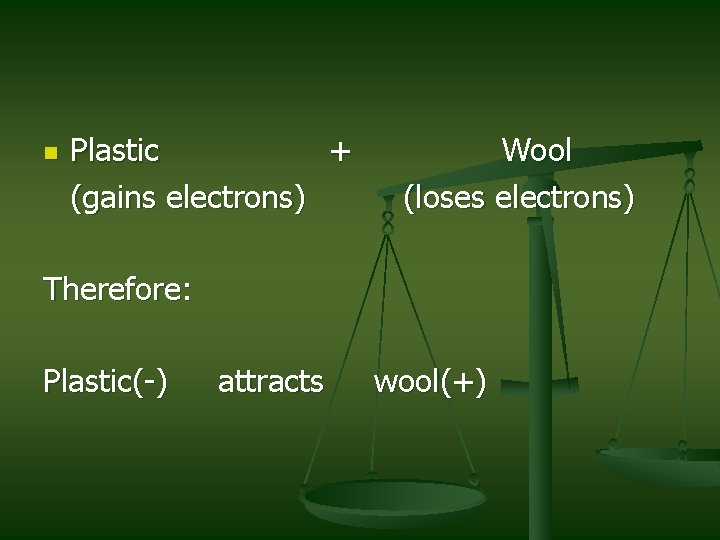
n Plastic + (gains electrons) Wool (loses electrons) Therefore: Plastic(-) attracts wool(+)

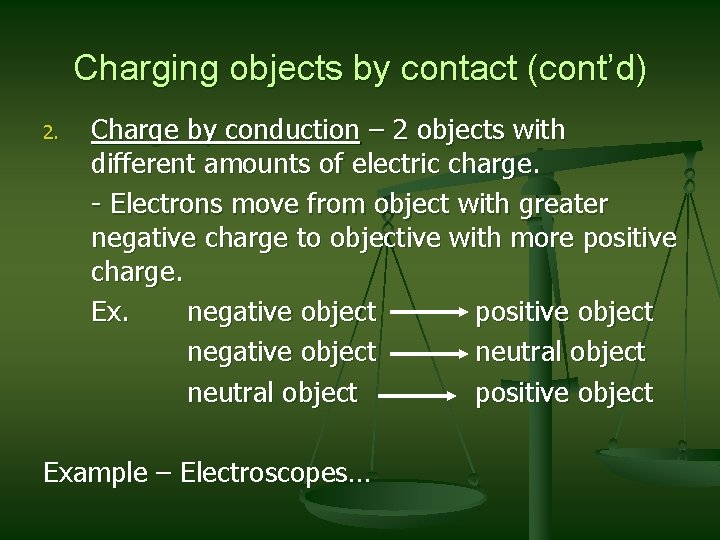
Charging objects by contact (cont’d) 2. Charge by conduction – 2 objects with different amounts of electric charge. - Electrons move from object with greater negative charge to objective with more positive charge. Ex. negative object positive object negative object neutral object positive object Example – Electroscopes…

Grounding n Connecting an object to a large body, like Earth, to neutralize the object’s charge. Give electrons to a positive object. n Remove electrons from a negative object. n Examples?