Static Electricity Static Electricity Two types of electricity












- Slides: 34

Static Electricity

Static Electricity Two types of electricity: current & STATIC Static Electricity is a build-up of charge in one place Non-moving

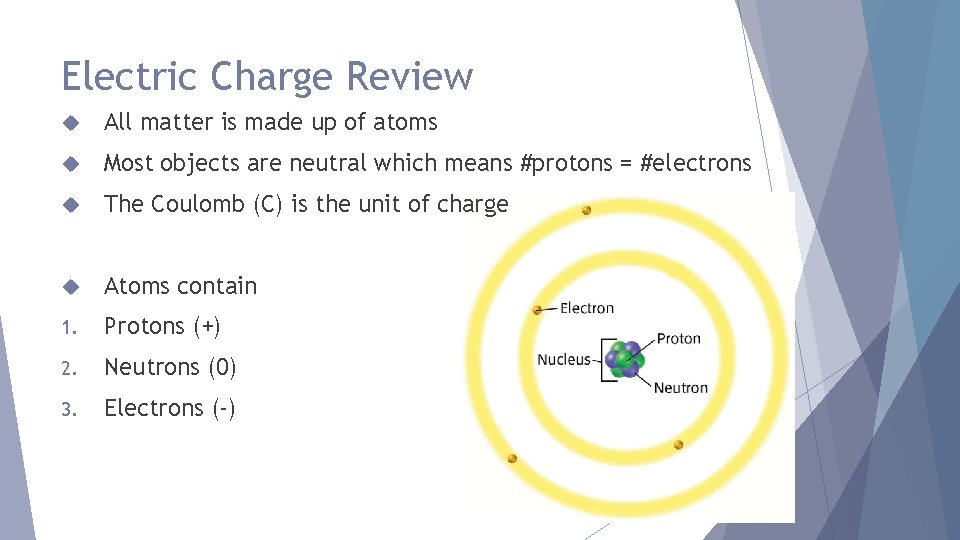
Electric Charge Review All matter is made up of atoms Most objects are neutral which means #protons = #electrons The Coulomb (C) is the unit of charge Atoms contain 1. Protons (+) 2. Neutrons (0) 3. Electrons (-)

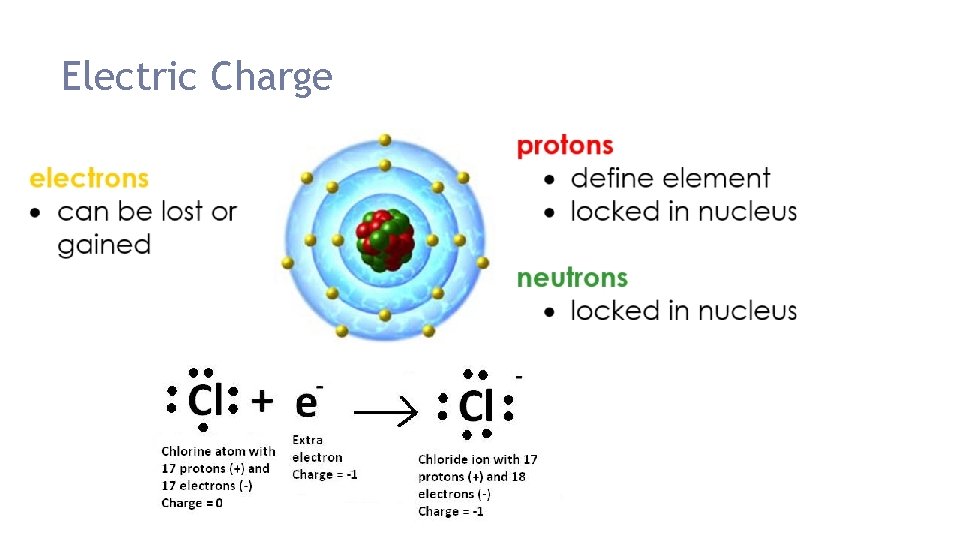
Electric Charge

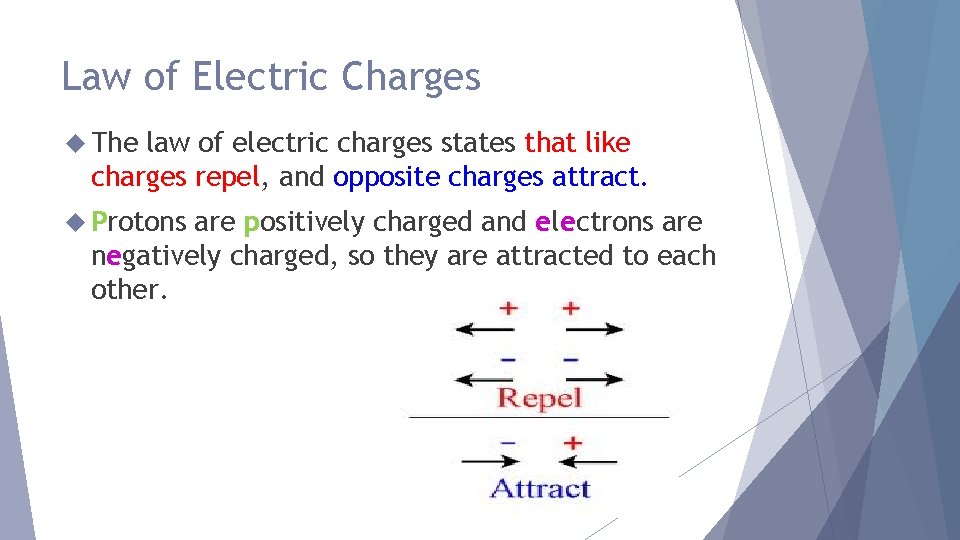
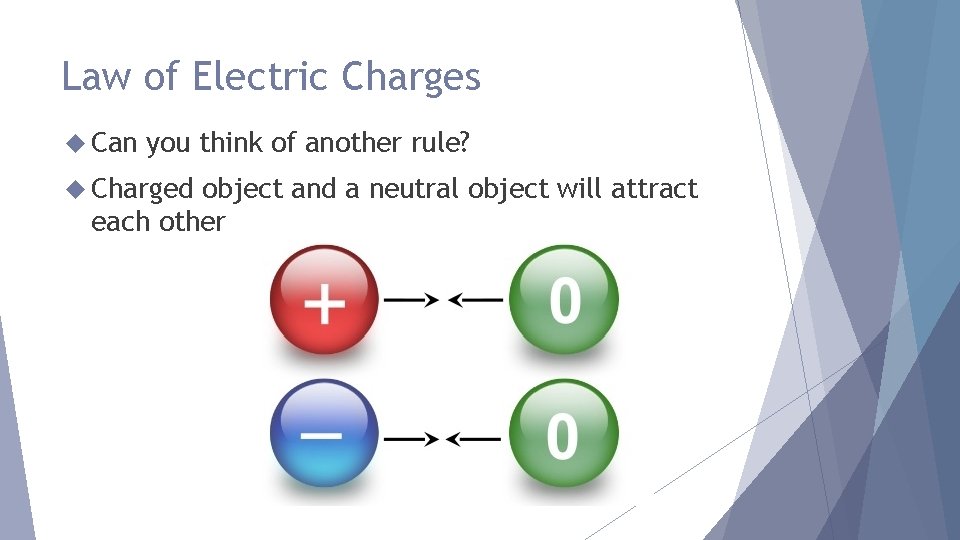
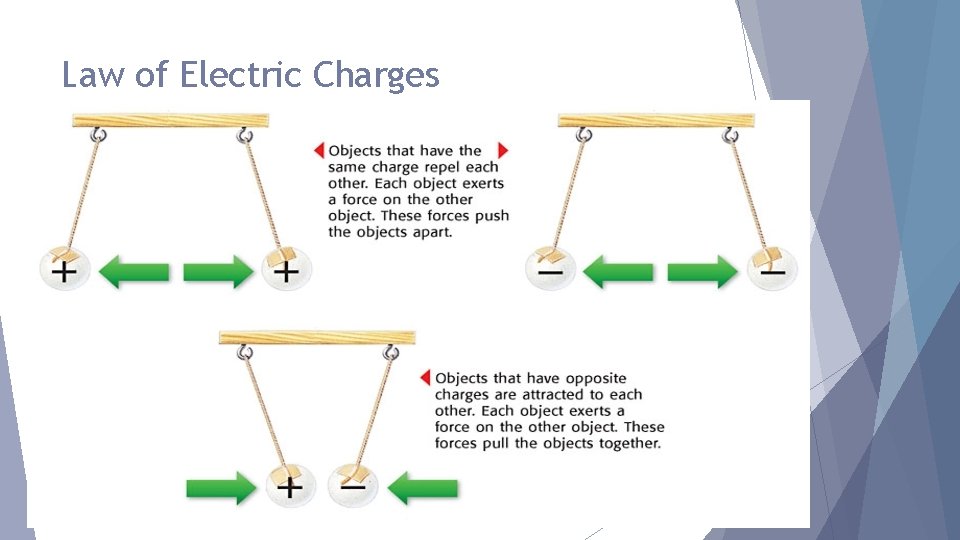
Law of Electric Charges The law of electric charges states that like charges repel, and opposite charges attract. Protons are positively charged and electrons are negatively charged, so they are attracted to each other.

Law of Electric Charges Can you think of another rule? Charged object and a neutral object will attract each other

Law of Electric Charges

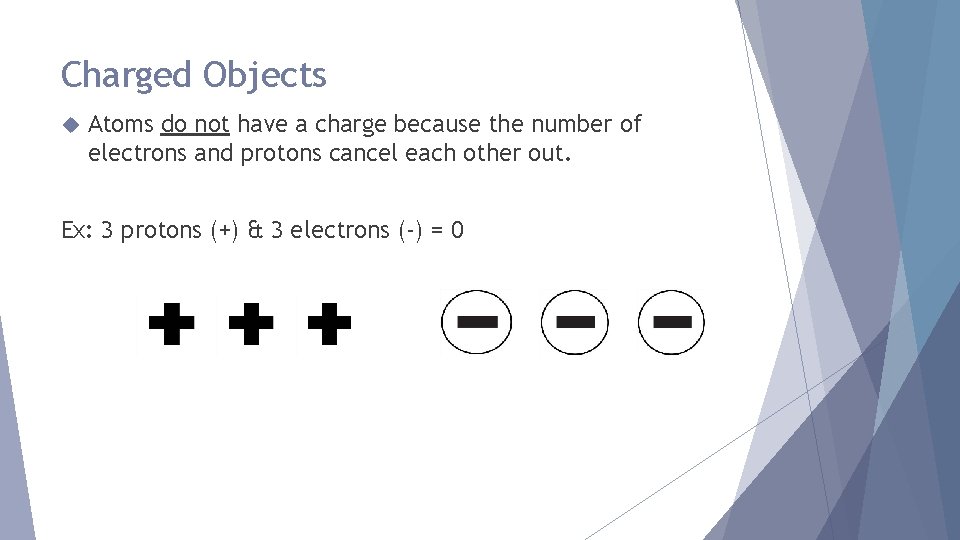
Charged Objects Atoms do not have a charge because the number of electrons and protons cancel each other out. Ex: 3 protons (+) & 3 electrons (-) = 0

Where do charges come from? If electrons = protons neutral If electrons > protons gained electrons, negative charge If electrons < protons lost electrons, positive charge

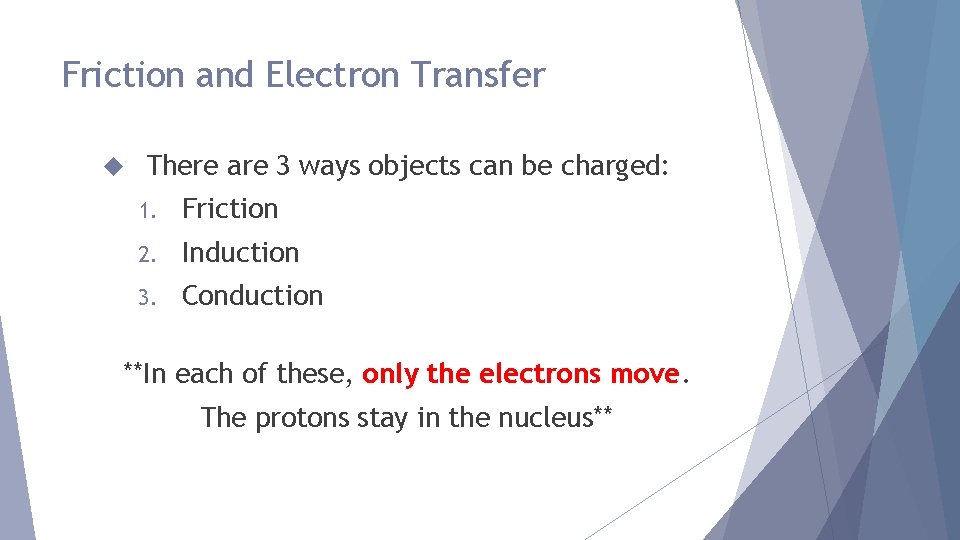
Friction and Electron Transfer There are 3 ways objects can be charged: 1. Friction 2. Induction 3. Conduction **In each of these, only the electrons move. The protons stay in the nucleus**

Friction Charging by friction occurs when electrons are transferred from one object onto another. Rubbing materials does not create electric charges. It just transfers electrons from one material to the other.

How Charging by Friction Works For example: Rubber has a much greater attraction for electrons than animal fur. As a result, the atoms of rubber pull e- from the atoms of animal fur This leaves both objects with an imbalance of charge: The rubber balloon has an excess of e- so it has a negative charge The animal fur has a shortage of e- which leaves it positively charged. The two objects have become charged with opposite types of charges as a result of the transfer of e- from the least electron-loving material to the most electron-loving material.

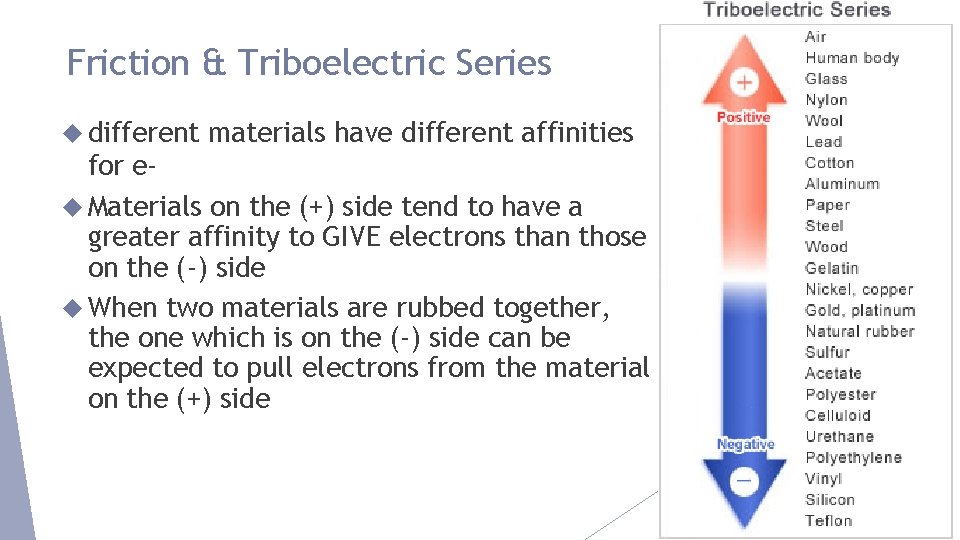
Friction & Triboelectric Series different materials have different affinities for e Materials on the (+) side tend to have a greater affinity to GIVE electrons than those on the (-) side When two materials are rubbed together, the one which is on the (-) side can be expected to pull electrons from the material on the (+) side

Become positive in charge The following materials tend to give up electrons when brought in contact with other materials. That means they will have an increase of positive (+) charges. The materials are listed with those that have the greatest tendency to give up electrons at the top to those that barely give up electrons. Materials that gain a positive (+) electrical charges (Tend to give up electrons) Most (+) charges Air Greatest tendency for giving up electrons and becoming highly positive (+) in charge Dry human skin Greatest tendency of a solid to give up electrons and becoming highly positive (+) in charge Leather Rabbit fur Fur is often used to create static electricity Glass The glass on your TV screen gets charged and collects dust Moderate (+) Human hair charges "Flyaway hair" is a good example of having a moderate positive (+) charge Nylon Wool Lead A surprise that lead would collect as much static electricity as cat fur Cat fur Silk Aluminum Least (+) Paper charges Gives up some electrons

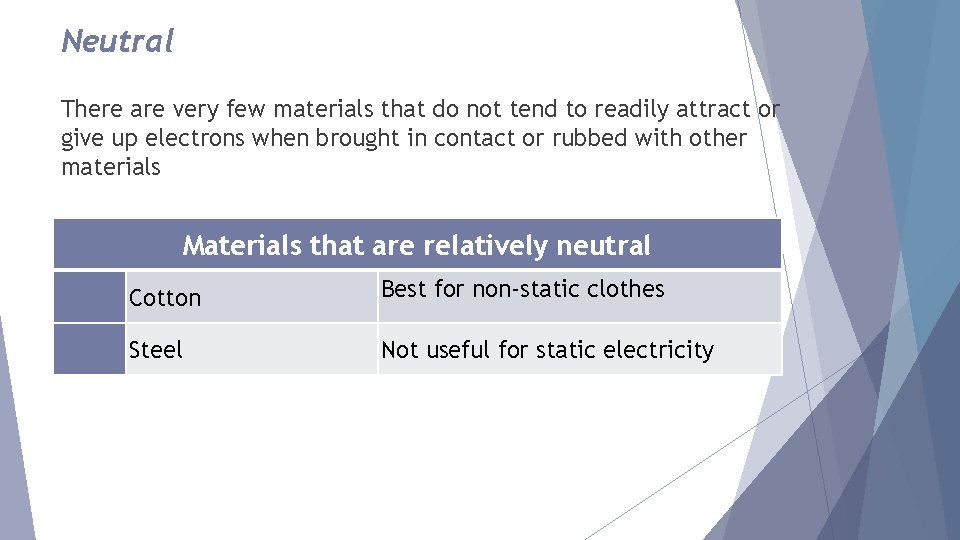
Neutral There are very few materials that do not tend to readily attract or give up electrons when brought in contact or rubbed with other materials Materials that are relatively neutral Cotton Best for non-static clothes Steel Not useful for static electricity

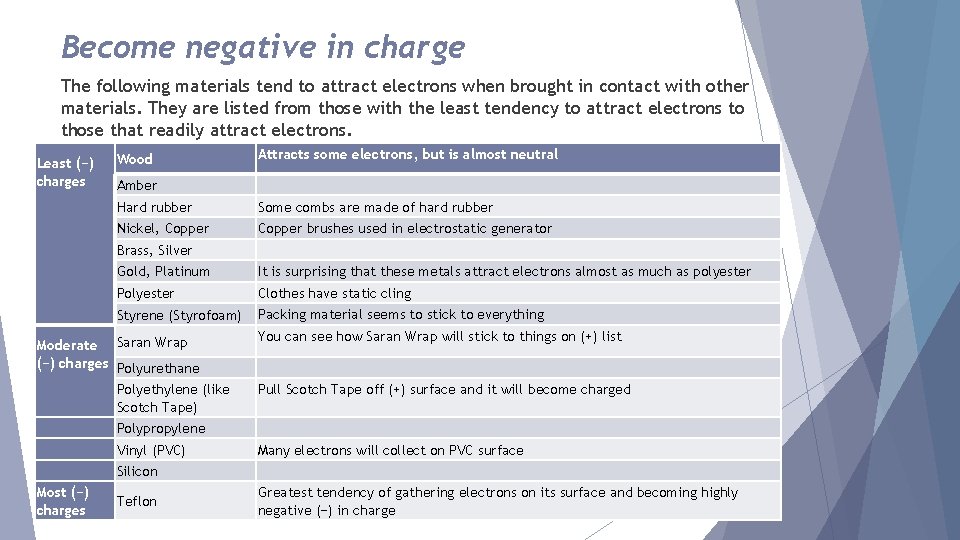
Become negative in charge The following materials tend to attract electrons when brought in contact with other materials. They are listed from those with the least tendency to attract electrons to those that readily attract electrons. Least (−) charges Wood Attracts some electrons, but is almost neutral Amber Hard rubber Some combs are made of hard rubber Nickel, Copper brushes used in electrostatic generator Brass, Silver Gold, Platinum It is surprising that these metals attract electrons almost as much as polyester Polyester Clothes have static cling Styrene (Styrofoam) Packing material seems to stick to everything Moderate Saran Wrap (−) charges Polyurethane Polyethylene (like Scotch Tape) You can see how Saran Wrap will stick to things on (+) list Pull Scotch Tape off (+) surface and it will become charged Polypropylene Vinyl (PVC) Many electrons will collect on PVC surface Silicon Most (−) charges Teflon Greatest tendency of gathering electrons on its surface and becoming highly negative (−) in charge

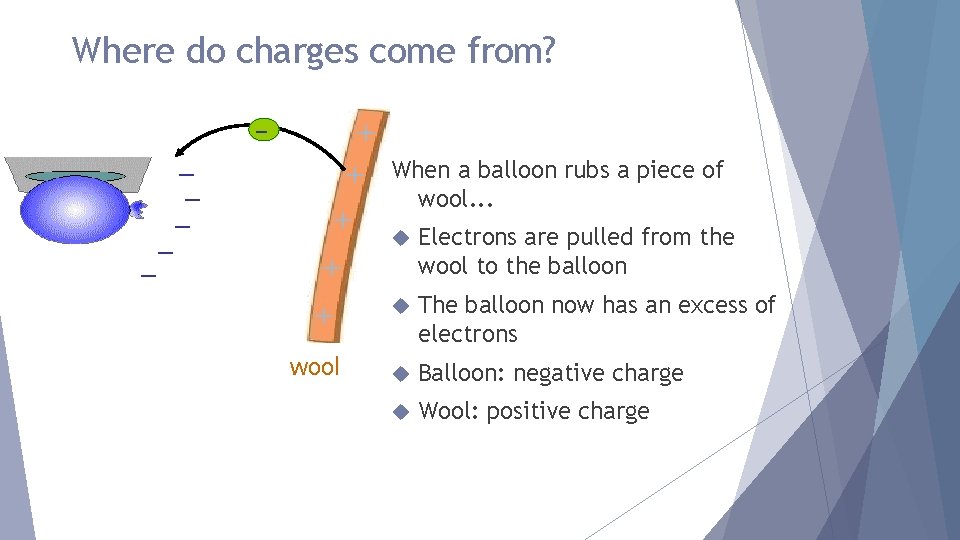
Where do charges come from? + + When a balloon rubs a piece of – – – + wool. . . Electrons are pulled from the wool to the balloon + The balloon now has an excess of electrons wool Balloon: negative charge Wool: positive charge +

Insulators An electrical insulator is a material in which charges cannot move easily. Insulators do not conduct charges very well because their electrons cannot flow freely. The electrons are tightly held in the atoms of the insulator. The insulating material in a lamp cord stops charges from leaving the wire and protects you from electric shock. Plastic, rubber, glass, wood, and air are good insulators.

Conductors An electrical conductor is a material in which charges can move easily. Most metals are good conductors because some of their electrons are free to move. Conductors are used to make wires. For example, a lamp cord has metal wire and metal prongs. Copper, aluminum, and mercury are good conductors

Balloon Simulation Why does a balloon stick to a wall?

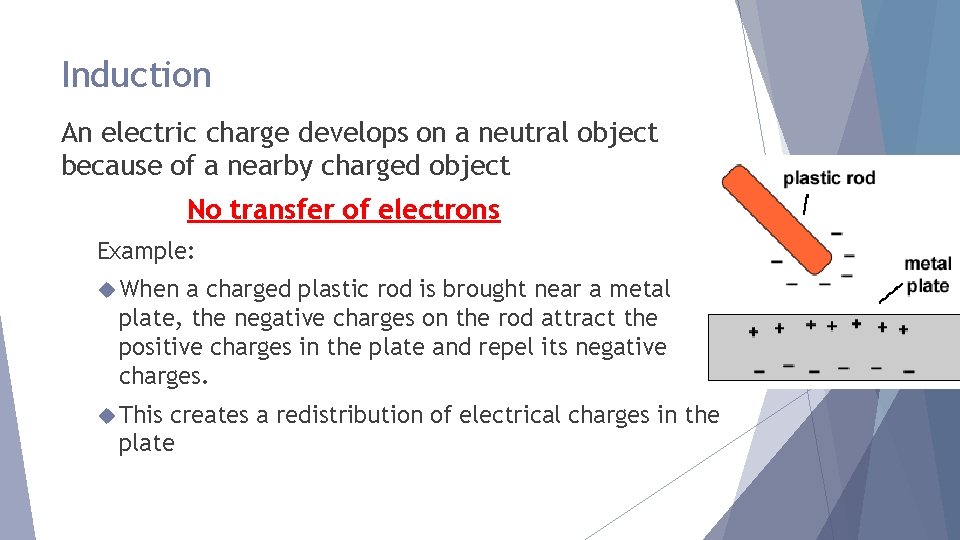
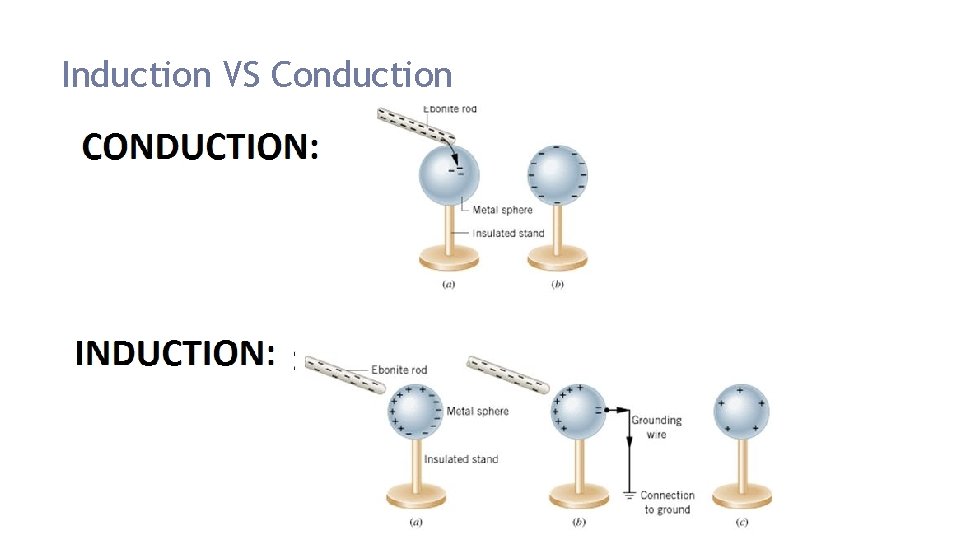
Induction An electric charge develops on a neutral object because of a nearby charged object No transfer of electrons Example: When a charged plastic rod is brought near a metal plate, the negative charges on the rod attract the positive charges in the plate and repel its negative charges. This creates a redistribution of electrical charges in the plate

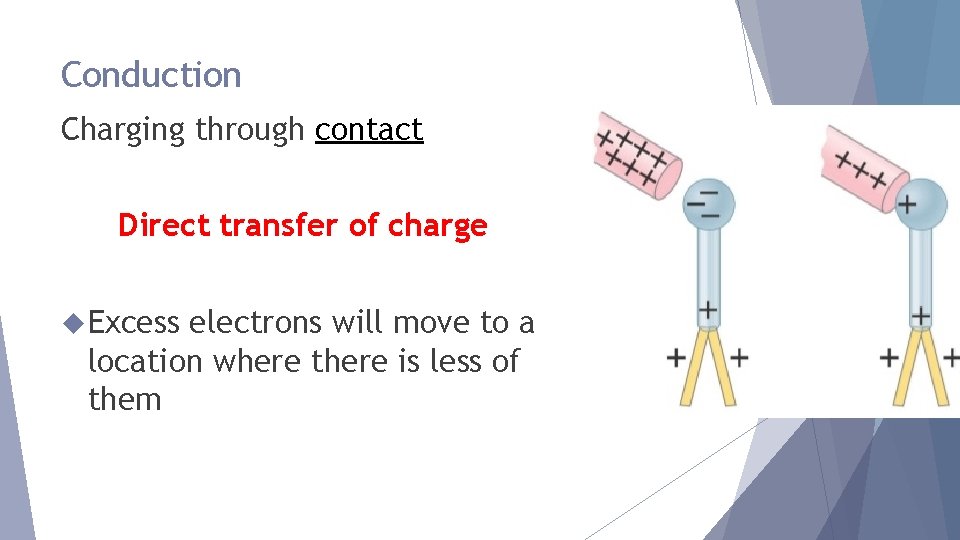
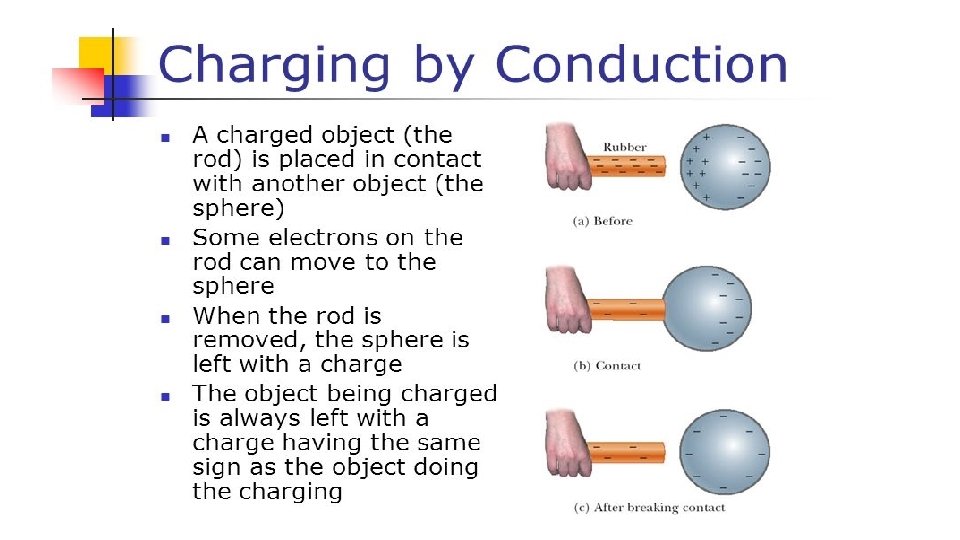
Conduction Charging through contact Direct transfer of charge Excess electrons will move to a location where there is less of them


Induction VS Conduction

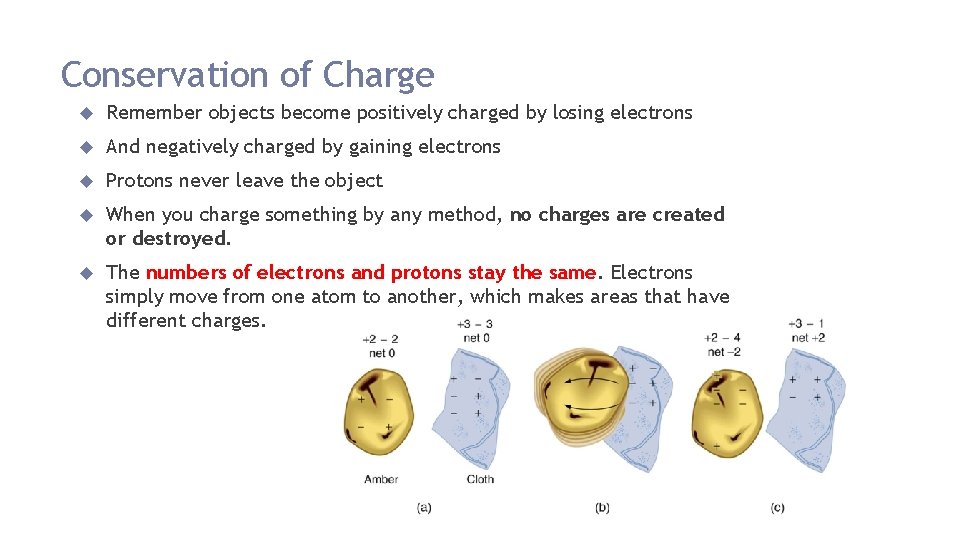
Conservation of Charge Remember objects become positively charged by losing electrons And negatively charged by gaining electrons Protons never leave the object When you charge something by any method, no charges are created or destroyed. The numbers of electrons and protons stay the same. Electrons simply move from one atom to another, which makes areas that have different charges.

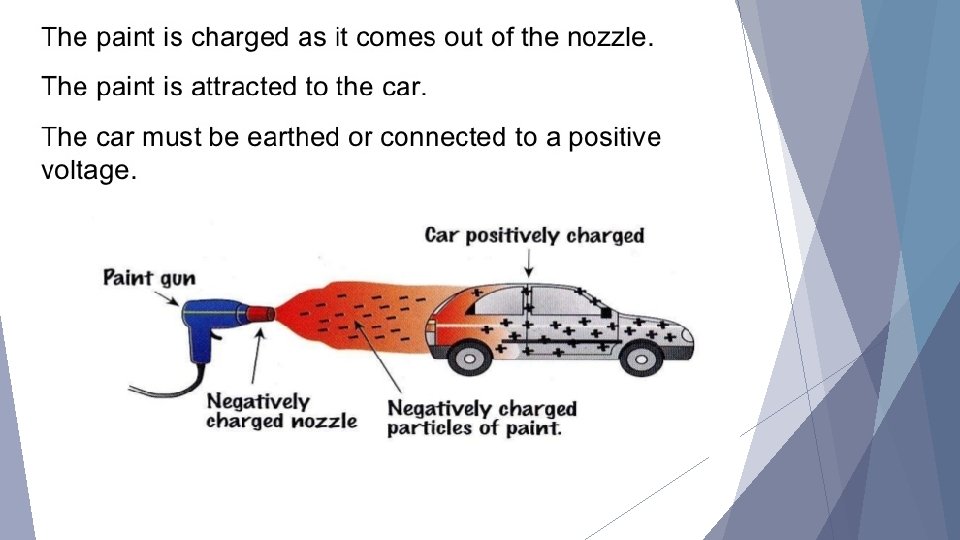
Applications and Dangers Static charge can be very useful - electrostatic filters can be used to clean air, paint automobiles, and hold objects with electrostatic attraction.


Applications and Dangers Static charge can also be dangerous - when charge builds up it can discharge and cause serious shocks, explosions or fires. Lightning is especially dangerous - buildings can be protected with lightning rods.

Electric Discharge The loss of static electricity as charges move off an object is called electric discharge. Sometimes, electric discharge happens slowly. Ex: static on clothes Sometimes, electric discharge happens quickly. Ex. wearing rubbersoled shoes on carpet, lightning

Thunderstorm Safety Avoid contact with corded phones and devices including those plugged in for recharging. Cordless and wireless phones not connected to wall outlets are OK to use. Avoid contact with electrical equipment or cords. Unplug appliances and other electrical items such as computers and turn off air conditioners. Why? Power surges from lightning can cause serious damage. Avoid contact with plumbing. Do not wash your hands, do not take a shower, do not wash dishes, and do not do laundry. Plumbing and bathroom fixtures can conduct electricity. Anything else you can think of?

Thunderstorm Safety Stay away from windows and doors, and stay off porches. Avoid hilltops, open fields, the beach or a boat on the water. Take shelter in a sturdy building. Avoid isolated sheds or other small structures in open areas. Avoid contact with anything metal—tractors, farm equipment, motorcycles, golf carts, golf clubs, and bicycles. If you are driving, try to safely exit the road and park. Stay in the vehicle and turn on the emergency flashers until the heavy rain ends. Avoid touching metal or other surfaces that conduct electricity in and outside the vehicle.

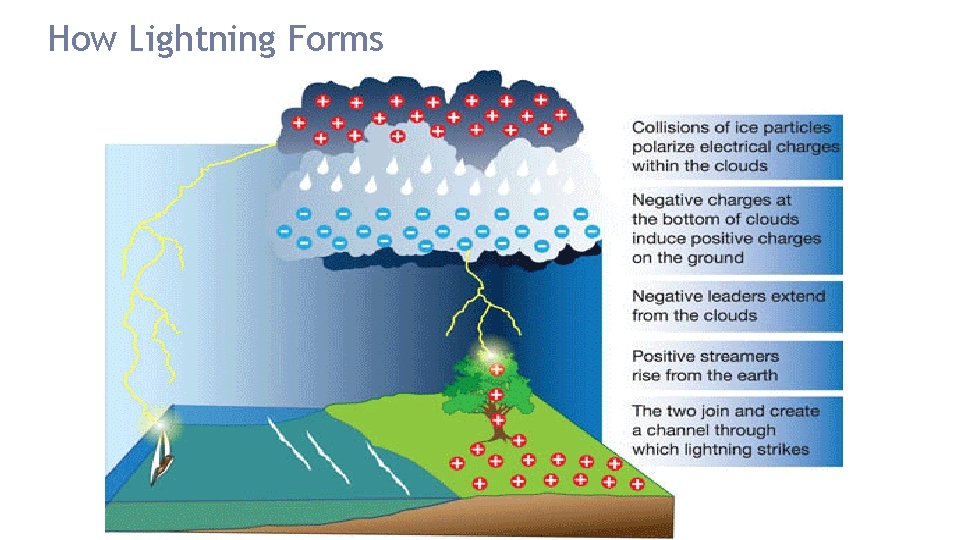
How Lightning Forms

Lightning usually strikes the highest point because that point provides the shortest path for the charges to reach the ground. Anything that sticks up or out in an area can provide a path for lightning. Objects, such as a lightning rod, that are joined to Earth by a conductor, such as a wire, are “grounded. ” Any object that is grounded provides a path for electric charges to move to Earth. Because Earth is so large, it can give up or absorb charges without being damaged.

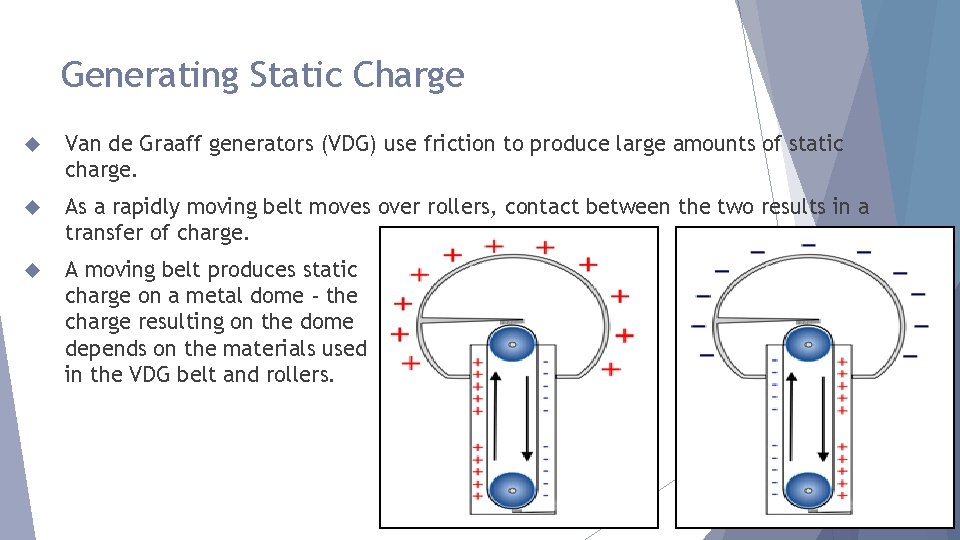
Generating Static Charge Van de Graaff generators (VDG) use friction to produce large amounts of static charge. As a rapidly moving belt moves over rollers, contact between the two results in a transfer of charge. A moving belt produces static charge on a metal dome - the charge resulting on the dome depends on the materials used in the VDG belt and rollers.