Understanding Predicting and Preventing Oilfield Scale This may





- Slides: 21

* Understanding, Predicting and Preventing Oilfield Scale

This may be the only time you get to review this part of oilfield chemistry, so we will go deeper than the numbers * The thermodynamic basis and the models currently in use

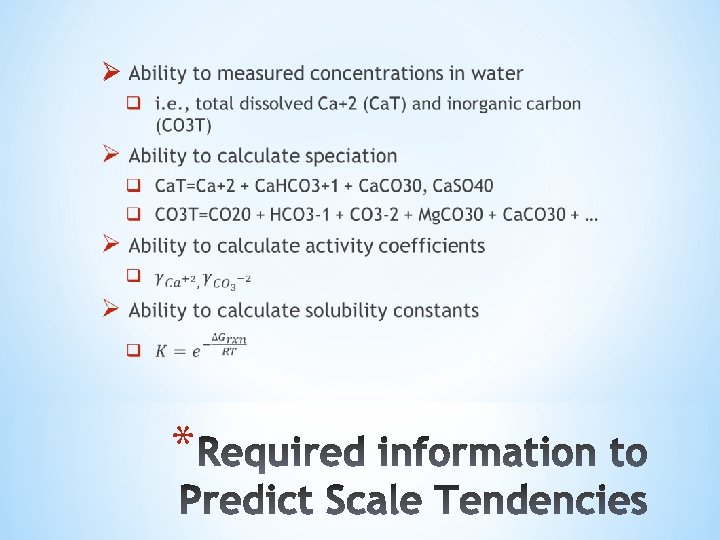
Ø Names used for S q Oilfield names o o Scale Tendency (ST), Scale Ratio (SR) Scale Index (SI) is log 10 value of S q Scientific names o Saturation Ratio (SR, ), Supersaturation (S) Ø S is thermodynamic driving force for solids to precipitate or dissolve q Supersaturated (S>1 or SI>0): precipitates spontaneously q Subsaturated (S<1 or SI<0): dissolves spontaneously q Saturated (S=1 or SI=0): neither dissolves nor precipitates *

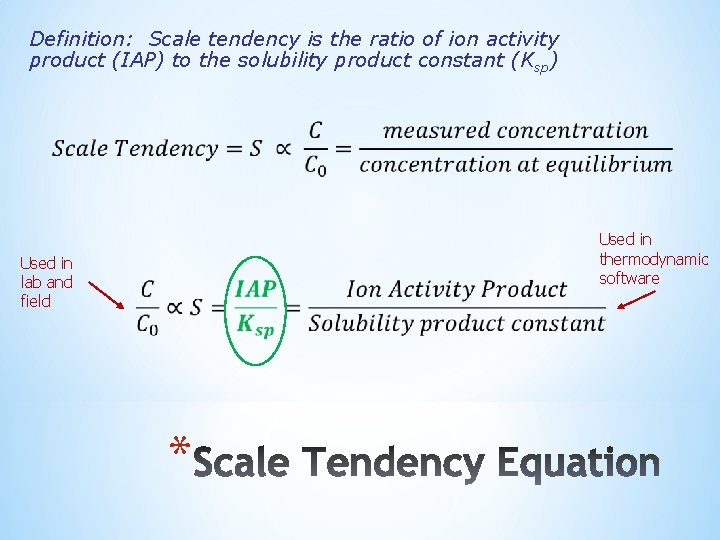
Definition: Scale tendency is the ratio of ion activity product (IAP) to the solubility product constant (Ksp) Used in lab and field Used in thermodynamic software *

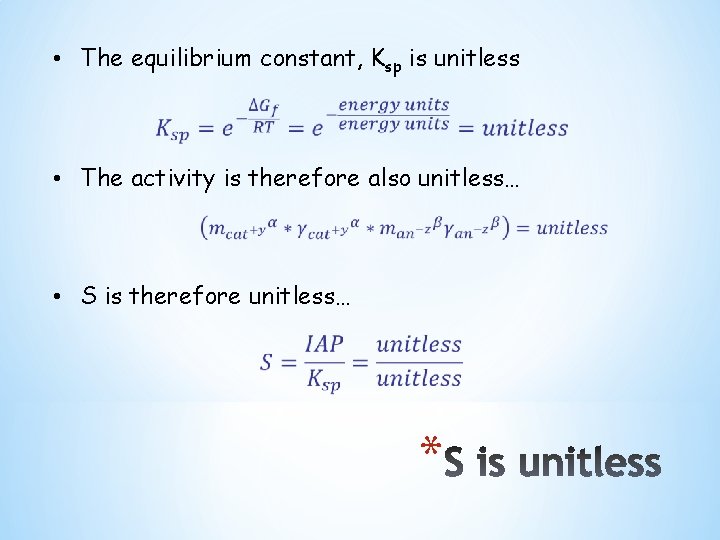
• The equilibrium constant, Ksp is unitless • The activity is therefore also unitless… • S is therefore unitless… *


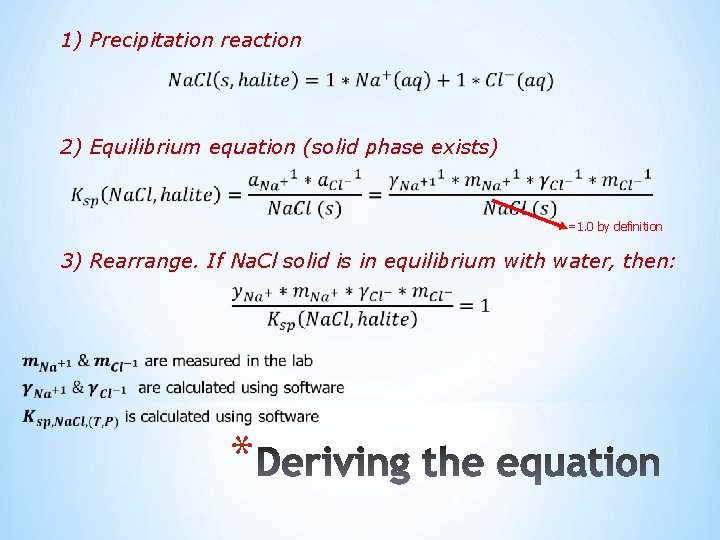
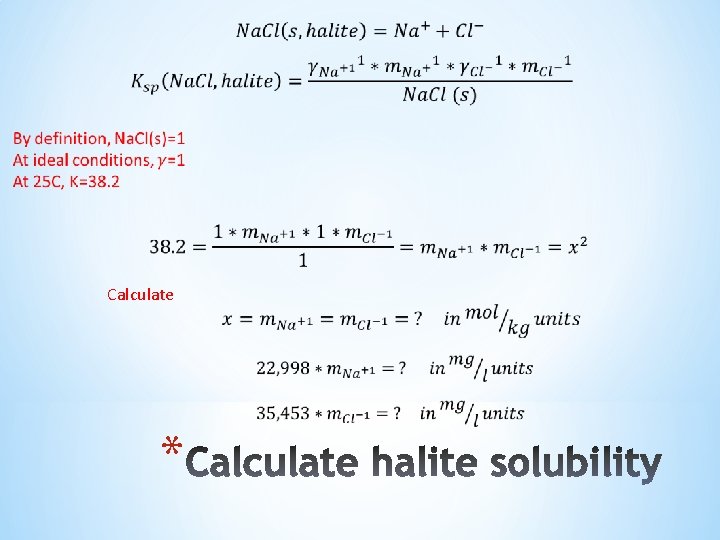
1) Precipitation reaction 2) Equilibrium equation (solid phase exists) =1. 0 by definition 3) Rearrange. If Na. Cl solid is in equilibrium with water, then: *

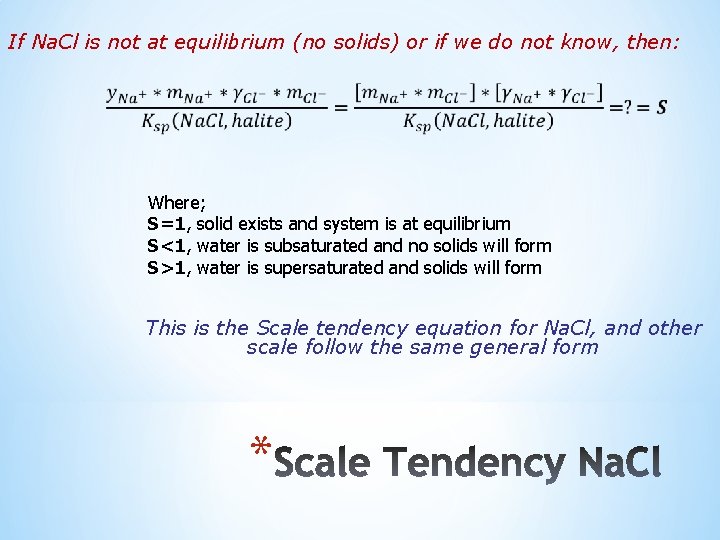
If Na. Cl is not at equilibrium (no solids) or if we do not know, then: Where; S=1, solid exists and system is at equilibrium S<1, water is subsaturated and no solids will form S>1, water is supersaturated and solids will form This is the Scale tendency equation for Na. Cl, and other scale follow the same general form *

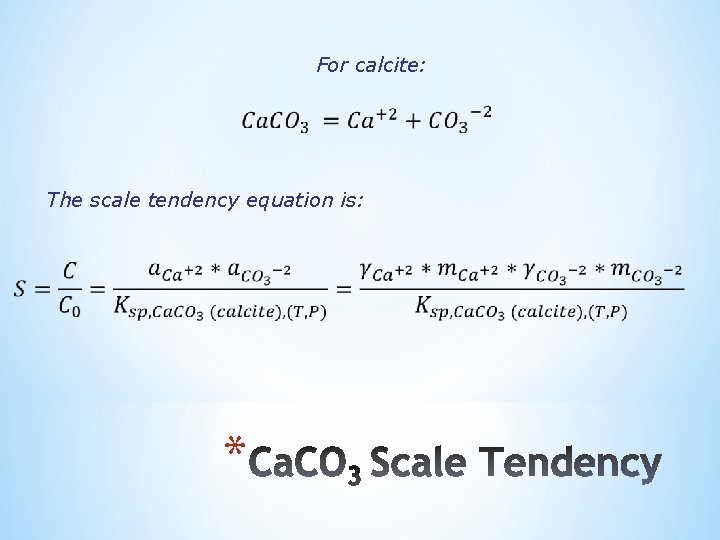
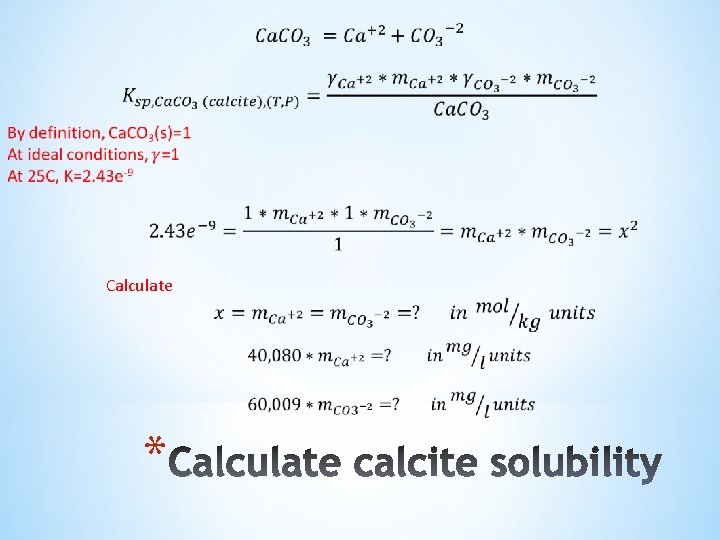
For calcite: The scale tendency equation is: *

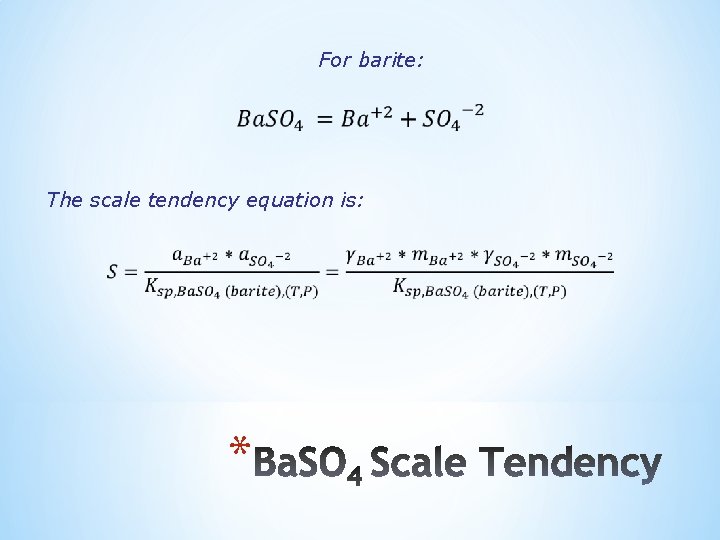
For barite: The scale tendency equation is: *

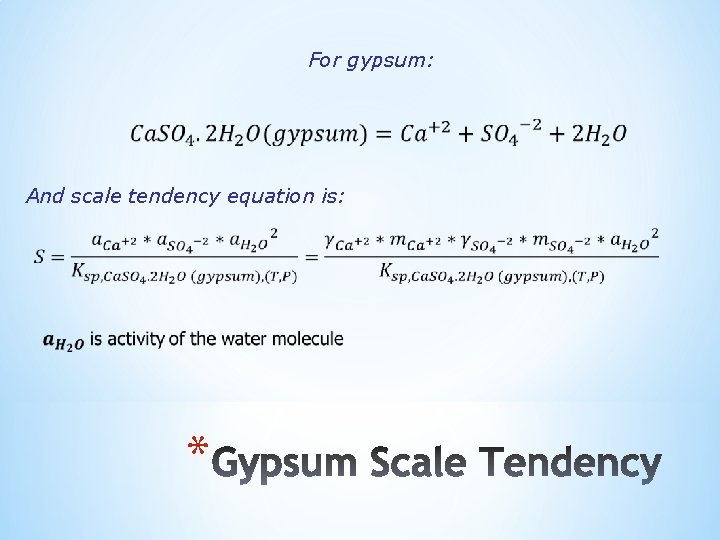
For gypsum: And scale tendency equation is: *

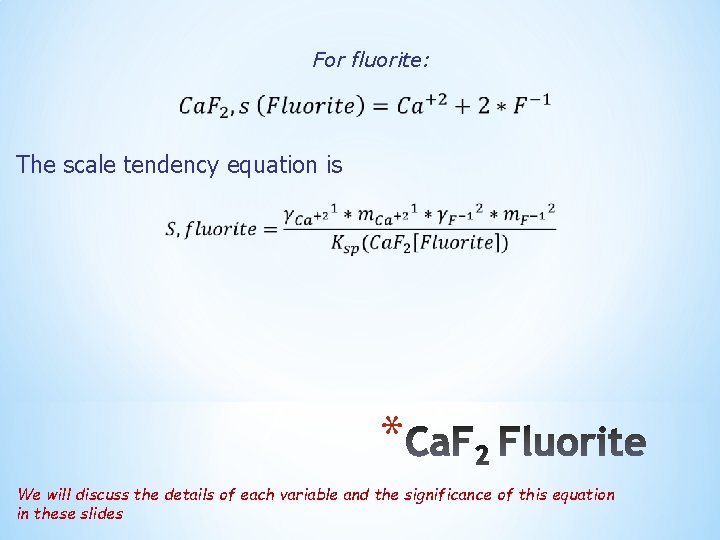
For fluorite: The scale tendency equation is * We will discuss the details of each variable and the significance of this equation in these slides

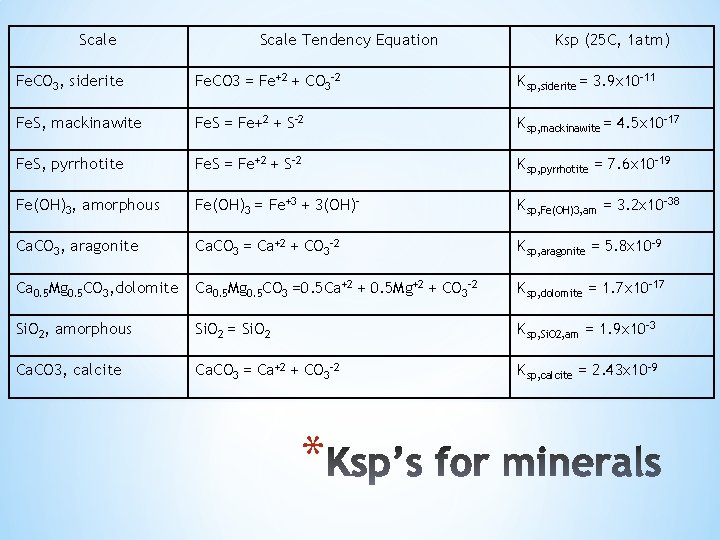
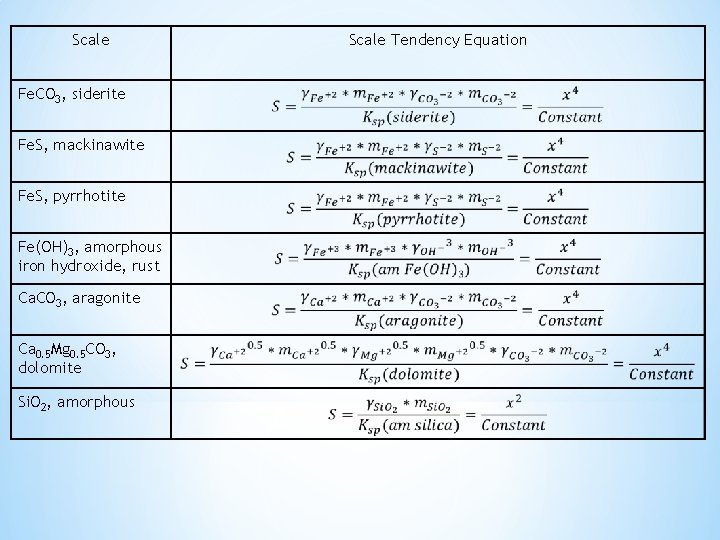
Scale Tendency Equation Ksp (25 C, 1 atm) Fe. CO 3, siderite Fe. CO 3 = Fe+2 + CO 3 -2 Ksp, siderite = 3. 9 x 10 -11 Fe. S, mackinawite Fe. S = Fe+2 + S-2 Ksp, mackinawite = 4. 5 x 10 -17 Fe. S, pyrrhotite Fe. S = Fe+2 + S-2 Ksp, pyrrhotite = 7. 6 x 10 -19 Fe(OH)3, amorphous Fe(OH)3 = Fe+3 + 3(OH)- Ksp, Fe(OH)3, am = 3. 2 x 10 -38 Ca. CO 3, aragonite Ca. CO 3 = Ca+2 + CO 3 -2 Ksp, aragonite = 5. 8 x 10 -9 Ca 0. 5 Mg 0. 5 CO 3, dolomite Ca 0. 5 Mg 0. 5 CO 3 =0. 5 Ca+2 + 0. 5 Mg+2 + CO 3 -2 Ksp, dolomite = 1. 7 x 10 -17 Si. O 2, amorphous Si. O 2 = Si. O 2 Ksp, Si. O 2, am = 1. 9 x 10 -3 Ca. CO 3, calcite Ca. CO 3 = Ca+2 + CO 3 -2 Ksp, calcite = 2. 43 x 10 -9 *

Calculate *

Calculate *

Calculate *

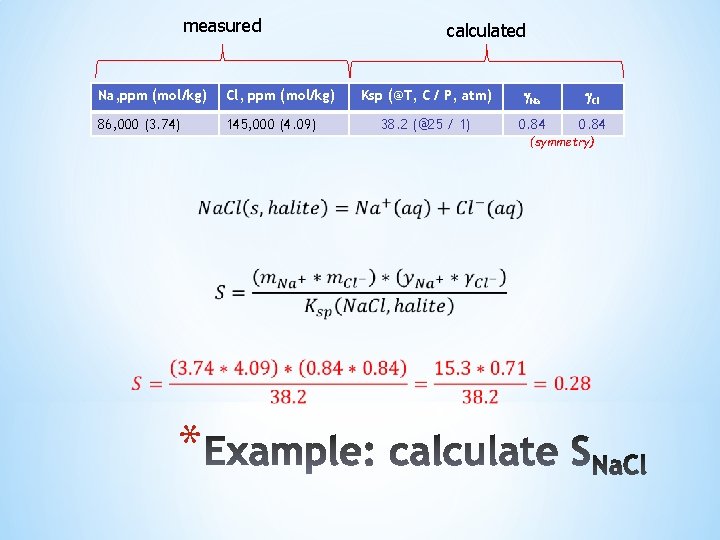
measured Na, ppm (mol/kg) Cl, ppm (mol/kg) 86, 000 (3. 74) 145, 000 (4. 09) calculated Ksp (@T, C / P, atm) Na Cl 38. 2 (@25 / 1) 0. 84 (symmetry) *

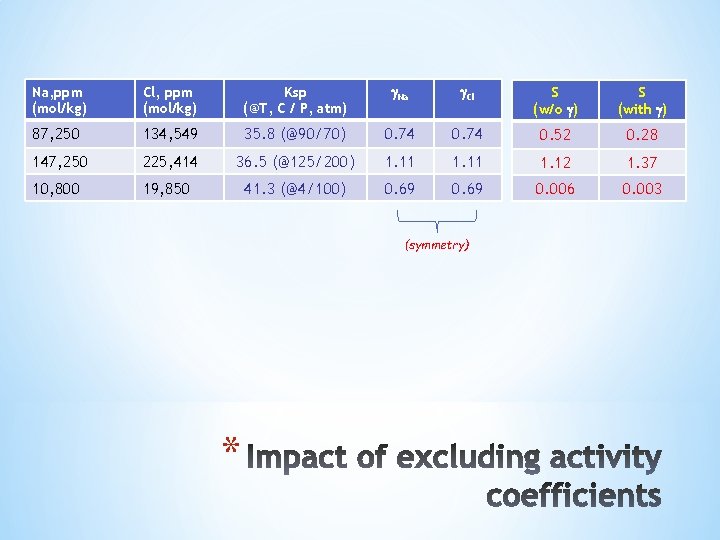
Na, ppm (mol/kg) Cl, ppm (mol/kg) Ksp (@T, C / P, atm) Na Cl S (w/o ) S (with ) 87, 250 134, 549 35. 8 (@90/70) 0. 74 0. 52 0. 28 147, 250 225, 414 36. 5 (@125/200) 1. 11 1. 12 1. 37 10, 800 19, 850 41. 3 (@4/100) 0. 69 0. 006 0. 003 (symmetry) *

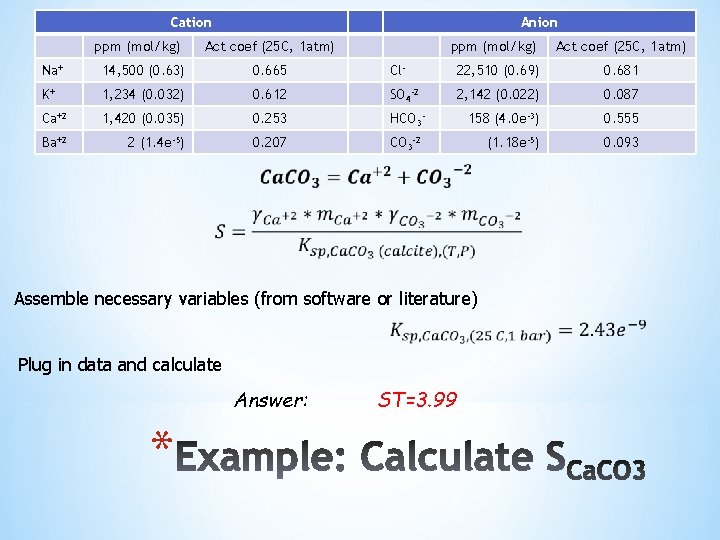
Cation ppm (mol/kg) Anion Act coef (25 C, 1 atm) ppm (mol/kg) Act coef (25 C, 1 atm) Na+ 14, 500 (0. 63) 0. 665 Cl- 22, 510 (0. 69) 0. 681 K+ 1, 234 (0. 032) 0. 612 SO 4 -2 2, 142 (0. 022) 0. 087 Ca+2 1, 420 (0. 035) 0. 253 HCO 3 - 158 (4. 0 e-3) 0. 555 Ba+2 2 (1. 4 e-5) 0. 207 CO 3 -2 (1. 18 e-5) 0. 093 Assemble necessary variables (from software or literature) Plug in data and calculate Answer: * ST=3. 99

Scale Fe. CO 3, siderite Fe. S, mackinawite Fe. S, pyrrhotite Fe(OH)3, amorphous iron hydroxide, rust Ca. CO 3, aragonite Ca 0. 5 Mg 0. 5 CO 3, dolomite Si. O 2, amorphous Scale Tendency Equation
