Transcranial Magnetic Stimulation Neurostar Treatment Device Patient Introduction








- Slides: 19

Transcranial Magnetic Stimulation Neurostar Treatment Device Patient Introduction Tariq J. Faridi, B. Sc, M. Ed. Director of Education and Research, Visual Odyssey Temple Georgia

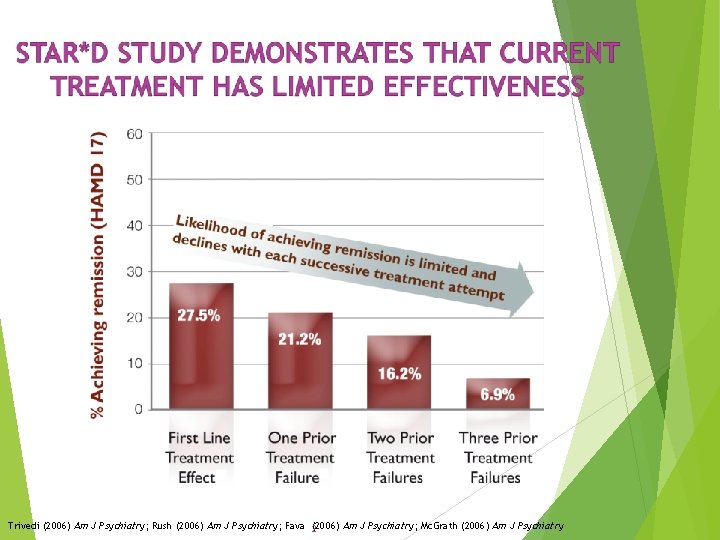
Trivedi (2006) Am J Psychiatry; Rush (2006) Am J Psychiatry; Fava (2006) Am J Psychiatry; Mc. Grath (2006) Am J Psychiatry 2

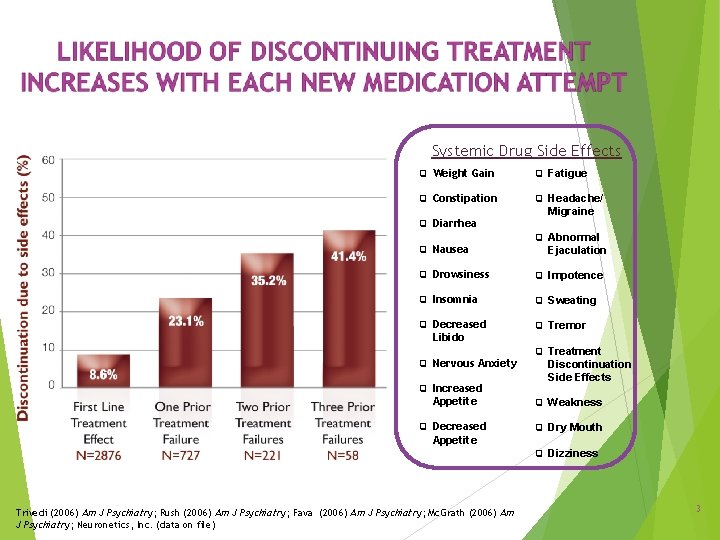
Systemic Drug Side Effects Weight Gain Fatigue Constipation Headache/ Diarrhea Nausea Migraine Abnormal Ejaculation Drowsiness Impotence Insomnia Sweating Decreased Tremor Libido Nervous Anxiety Increased Treatment Discontinuation Side Effects Appetite Weakness Decreased Dry Mouth Appetite Trivedi (2006) Am J Psychiatry; Rush (2006) Am J Psychiatry; Fava (2006) Am J Psychiatry; Mc. Grath (2006) Am J Psychiatry; Neuronetics, Inc. (data on file) Dizziness 3

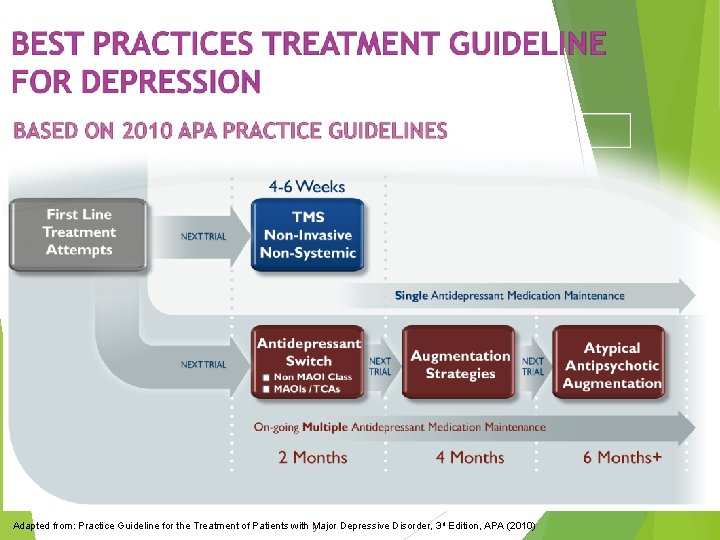
“The Neuro. Star TMS System is indicated for the treatment of adult patients with Major Depressive Disorder (MDD) who have failed to receive satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode…” Nearly all patients received multiple ineffective treatment attempts in current episode (range: 1 to 23 attempts, avg: 4) Demitrack & Thase. (2009) Psychopharm Bull

Adapted from: Practice Guideline for the Treatment of Patients with 5 Major Depressive Disorder, 3 rd Edition, APA (2010)

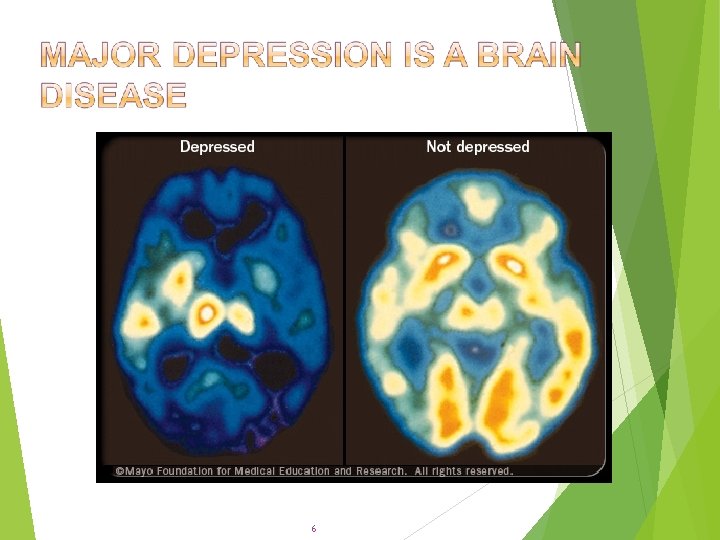
6

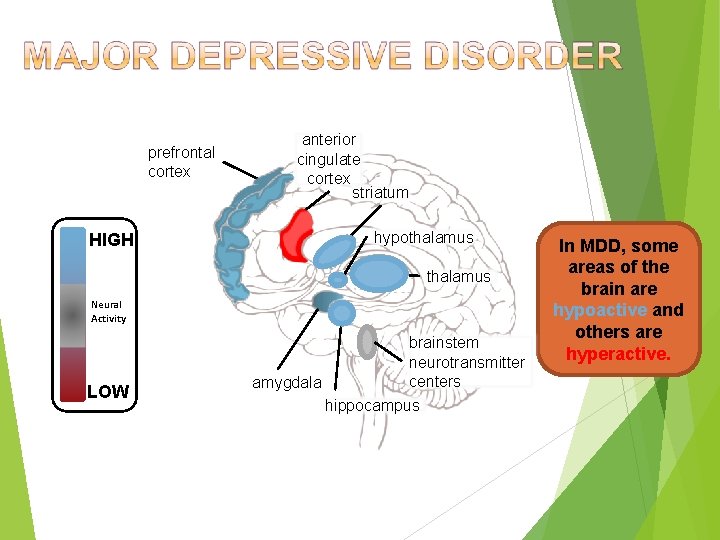
prefrontal cortex HIGH anterior cingulate cortex striatum hypothalamus Neural Activity LOW brainstem neurotransmitter centers amygdala hippocampus In MDD, some areas of the brain are hypoactive and others are hyperactive.

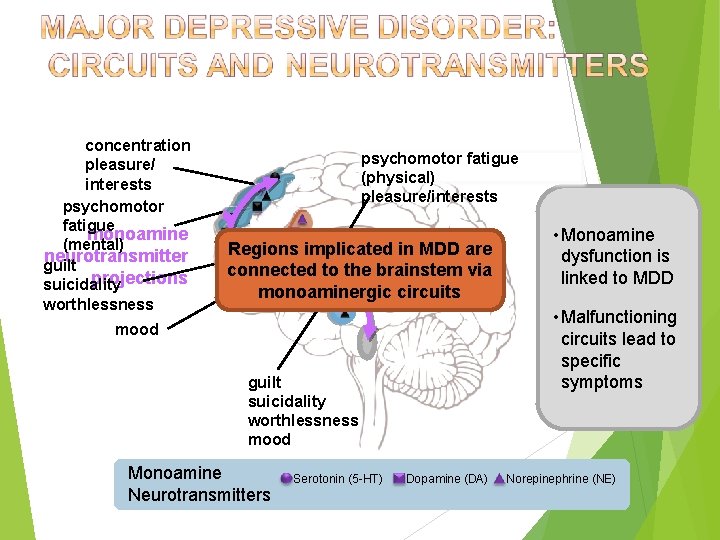
concentration pleasure/ interests psychomotor fatigue monoamine (mental) neurotransmitter guilt projections suicidality worthlessness psychomotor fatigue (physical) pleasure/interests sleep are Regions implicated in MDD appetite connected to the brainstem via monoaminergic circuits mood guilt suicidality worthlessness mood Monoamine Neurotransmitters Serotonin (5 -HT) Dopamine (DA) When there is appropriate • an Monoamine amount ofis dysfunction monoamine linked to MDD neurotransmitter • Malfunctioning activity, circuits lead to neuronal activity specific throughout the symptoms brain functions normally. Norepinephrine (NE)

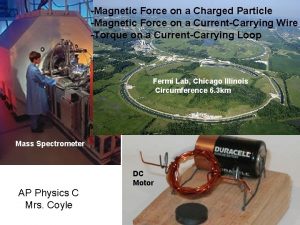
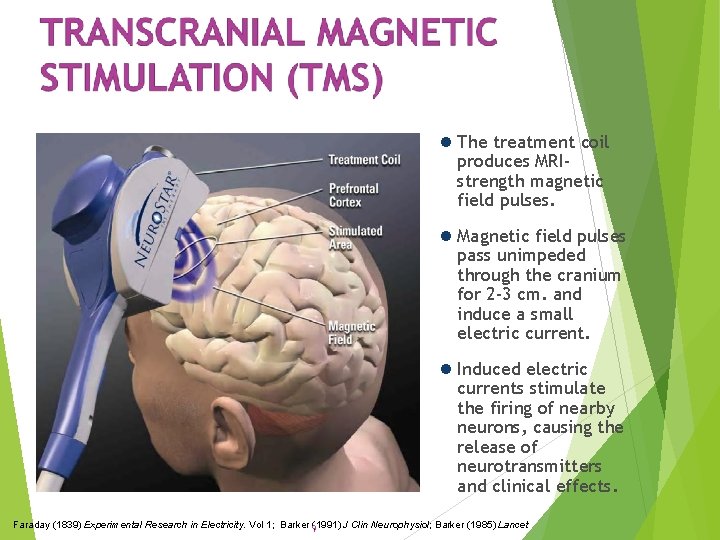
The treatment coil produces MRIstrength magnetic field pulses. Magnetic field pulses pass unimpeded through the cranium for 2 -3 cm. and induce a small electric current. Induced electric currents stimulate the firing of nearby neurons, causing the release of neurotransmitters and clinical effects. Faraday (1839) Experimental Research in Electricity. Vol 1; Barker 9(1991) J Clin Neurophysiol; Barker (1985) Lancet

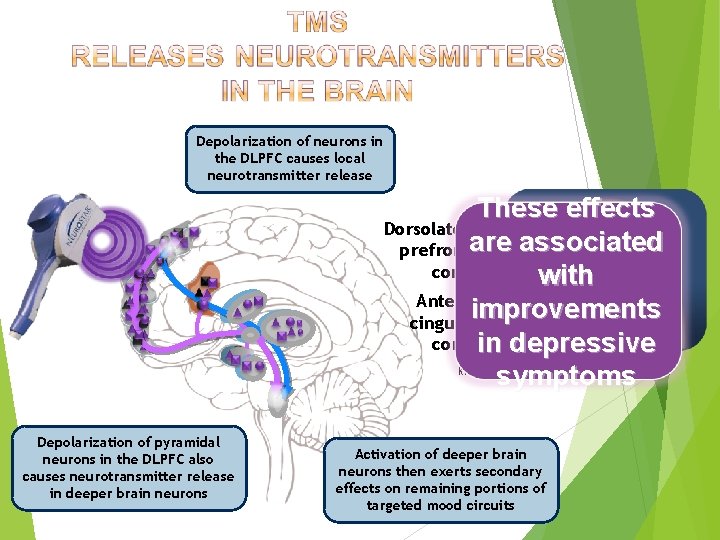
Depolarization of neurons in the DLPFC causes local neurotransmitter release These effects Dorsolateral are associated prefrontal cortex with Anterior improvements cingulate cortex in depressive symptoms Kito (2008) J Neuropsychiatry Clin Neurosci Depolarization of pyramidal neurons in the DLPFC also causes neurotransmitter release in deeper brain neurons Activation of deeper brain neurons then exerts secondary effects on remaining portions of targeted mood circuits

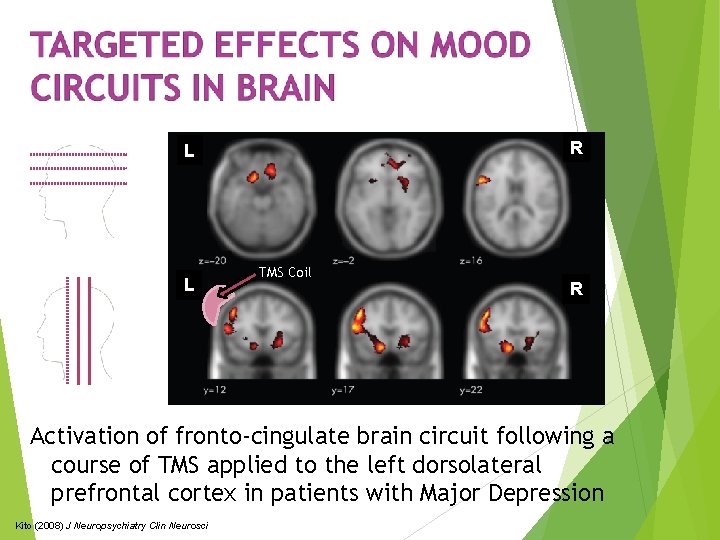
R L L TMS Coil R Activation of fronto-cingulate brain circuit following a course of TMS applied to the left dorsolateral prefrontal cortex in patients with Major Depression Kito (2008) J Neuropsychiatry Clin Neurosci


Only TMS device FDA-cleared for the treatment of depression Non-invasive and non-systemic The most common side effect associated with treatment is scalp pain or discomfort – generally mild to moderate Outpatient procedure, can be performed in a psychiatrist’s office; no anesthesia or sedation 37 minute treatment, administered daily for 4 -6 weeks Observed therapy facilitates adherence with treatment Available by prescription only 13

No systemic side effects No adverse effect on cognition Most common adverse event associated with treatment was scalp pain or discomfort < 5% of patients discontinued due to adverse events No seizures with TMS during clinical studies (over 10, 000 treatments) Six seizures reported with TMS in post-marketing period (estimated risk of seizure < 0. 1% per acute treatment course) Long term safety demonstrated in 6 months follow-up Janicak, et al. J Clin Psychiatry, 2008; Janicak, et al. Brain Stimulation, 2010.


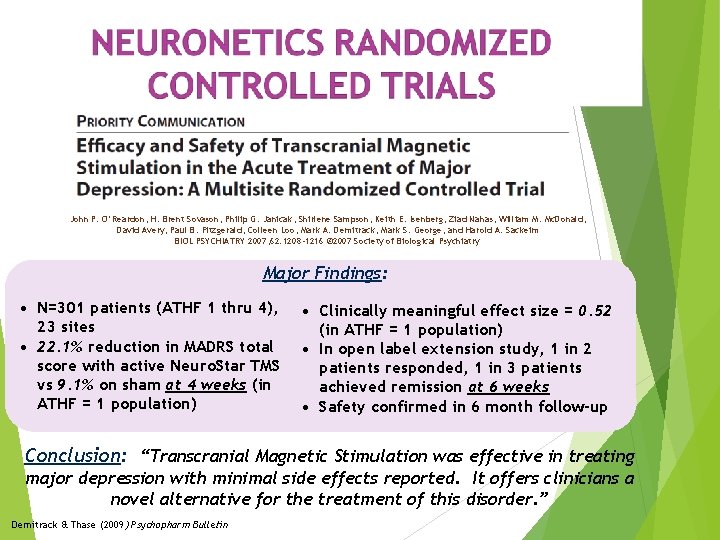
John P. O’Reardon, H. Brent Sovason, Philip G. Janicak, Shirlene Sampson, Keith E. Isenberg, Ziad Nahas, William M. Mc. Donald, David Avery, Paul B. Fitzgerald, Colleen Loo, Mark A. Demitrack, Mark S. George, and Harold A. Sackeim BIOL PSYCHIATRY 2007; 62: 1208 -1216 © 2007 Society of Biological Psychiatry Major Findings: • N=301 patients (ATHF 1 thru 4), 23 sites • 22. 1% reduction in MADRS total score with active Neuro. Star TMS vs 9. 1% on sham at 4 weeks (in ATHF = 1 population) • Clinically meaningful effect size = 0. 52 (in ATHF = 1 population) • In open label extension study, 1 in 2 patients responded, 1 in 3 patients achieved remission at 6 weeks • Safety confirmed in 6 month follow-up Conclusion: “Transcranial Magnetic Stimulation was effective in treating major depression with minimal side effects reported. It offers clinicians a novel alternative for the treatment of this disorder. ” Demitrack & Thase (2009) Psychopharm Bulletin

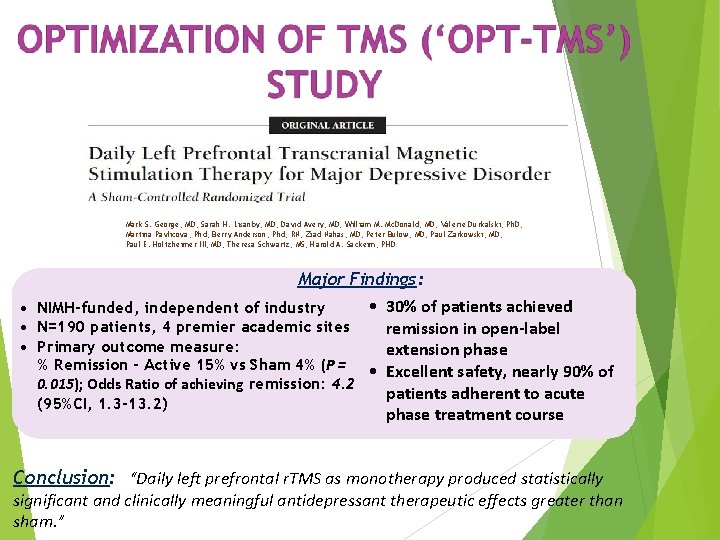
Mark S. George, MD; Sarah H. Lisanby, MD; David Avery, MD; William M. Mc. Donald, MD; Valerie Durkalski, Ph. D; Martina Pavlicova, Phd; Berry Anderson, Phd, RN; Ziad Nahas, MD; Peter Bulow, MD; Paul Zarkowski, MD; Paul E. Holtzheimer III, MD; Theresa Schwartz, MS; Harold A. Sackeim, PHD Major Findings: • 30% of patients achieved • NIMH-funded, independent of industry • N=190 patients, 4 premier academic sites remission in open-label • Primary outcome measure: extension phase % Remission - Active 15% vs Sham 4% (P = • Excellent safety, nearly 90% of 0. 015); Odds Ratio of achieving remission: 4. 2 patients adherent to acute (95%CI, 1. 3 -13. 2) phase treatment course Conclusion: “Daily left prefrontal r. TMS as monotherapy produced statistically significant and clinically meaningful antidepressant therapeutic effects greater than sham. ”

The evidence for the clinical efficacy of TMS in the treatment of depression is considerable, spanning more than 30 controlled clinical research studies Most recent meta-analysis (Slotema, et al, 2010): Included analysis of 34 studies involving 1, 383 patients Estimated standardized effect size = 0. 55 (P < 0. 001) Conclusion: “…r. TMS deserves a place in the standard toolbox of psychiatric treatment methods, as it is effective for depression …and has a mild side effect profile…. ” Slotema, et al. J Clin Psych (2010)

• Safety confirmed during long term, open-label 6 month follow up period – During open-label follow up on antidepressant medication monotherapy, ~37% of patients required TMS reintroduction – ~85% of patients who received TMS reintroduction benefited • Net incidence of illness relapse under these open-label follow up conditions: 11% – Six-month relapse with antidepressant treatment alone in STAR*D study was 35 -50% (Level 2 and 3 range) Janicak, et al. Brain Stimulation, 2010.
 Transcranial magnetic stimulation
Transcranial magnetic stimulation Carotid doppler velocity chart
Carotid doppler velocity chart Internal input devices
Internal input devices Confidential
Confidential Unit of magnetic flux is weber
Unit of magnetic flux is weber Magnetic moment and magnetic field relation
Magnetic moment and magnetic field relation Force on charged particle
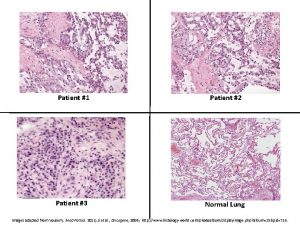
Force on charged particle Patient 2 patient
Patient 2 patient Drum printer definition
Drum printer definition Introduction to input devices
Introduction to input devices Is magnetic tape a secondary storage device
Is magnetic tape a secondary storage device Magnetic stripe reader input or output
Magnetic stripe reader input or output Cognitive stimulation therapy training
Cognitive stimulation therapy training Discovery learning adalah
Discovery learning adalah Noxious stimulation
Noxious stimulation Mogilner implants
Mogilner implants Integral stimulation
Integral stimulation Is phototropism external or internal
Is phototropism external or internal Cortisol stimulation test results
Cortisol stimulation test results Crh stimulation test interpretation
Crh stimulation test interpretation