Thermodynamics Free Energy When a system changes energy




- Slides: 9

Thermodynamics

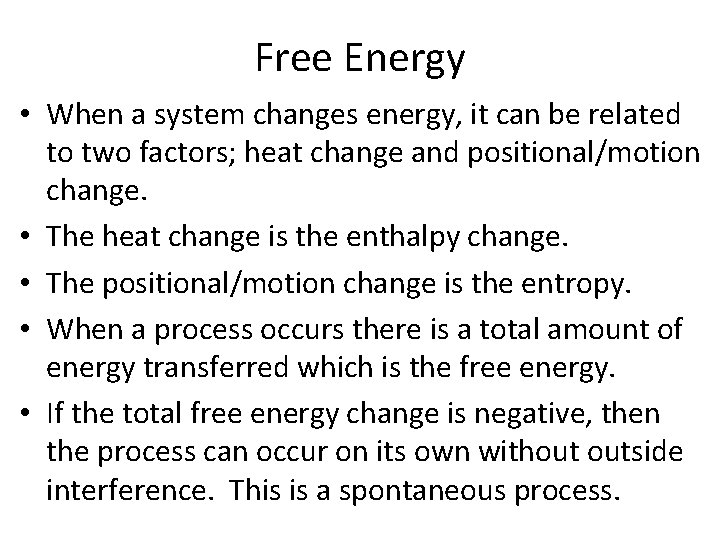
Free Energy • When a system changes energy, it can be related to two factors; heat change and positional/motion change. • The heat change is the enthalpy change. • The positional/motion change is the entropy. • When a process occurs there is a total amount of energy transferred which is the free energy. • If the total free energy change is negative, then the process can occur on its own without outside interference. This is a spontaneous process.

Spontaneity • If a process is spontaneous in one direction, then it is non spontaneous in the reverse direction. • Thermodynamic properties tell us nothing about the rate at which the reaction occurs. • Reversible processes are processes that can be restored to their original state exactly by reversing the process. • All spontaneous reactions are irreversible since it takes work from the surroundings to get it back.

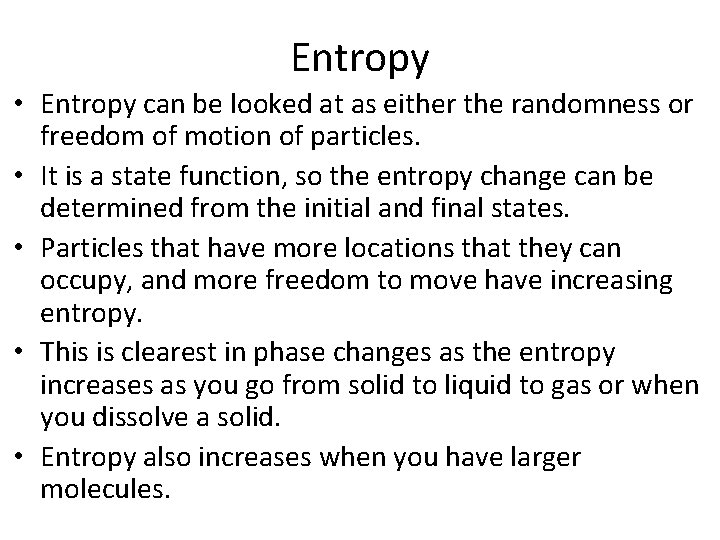
Entropy • Entropy can be looked at as either the randomness or freedom of motion of particles. • It is a state function, so the entropy change can be determined from the initial and final states. • Particles that have more locations that they can occupy, and more freedom to move have increasing entropy. • This is clearest in phase changes as the entropy increases as you go from solid to liquid to gas or when you dissolve a solid. • Entropy also increases when you have larger molecules.

Free energy and Entropy • When ∆G is negative, it means there is a release of energy. • That energy goes into the universe raising the randomness of the universe. • If the process is reversible then there is no net change to the universe so your free energy change is zero. • The second law of thermodynamics states that the entropy of the universe is unchanged in a reversible process, and increases for an irreversible process.

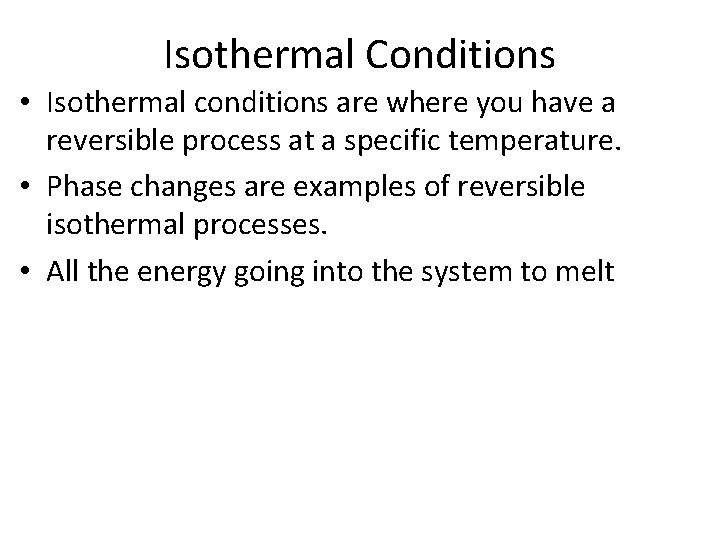
Isothermal Conditions • Isothermal conditions are where you have a reversible process at a specific temperature. • Phase changes are examples of reversible isothermal processes. • All the energy going into the system to melt

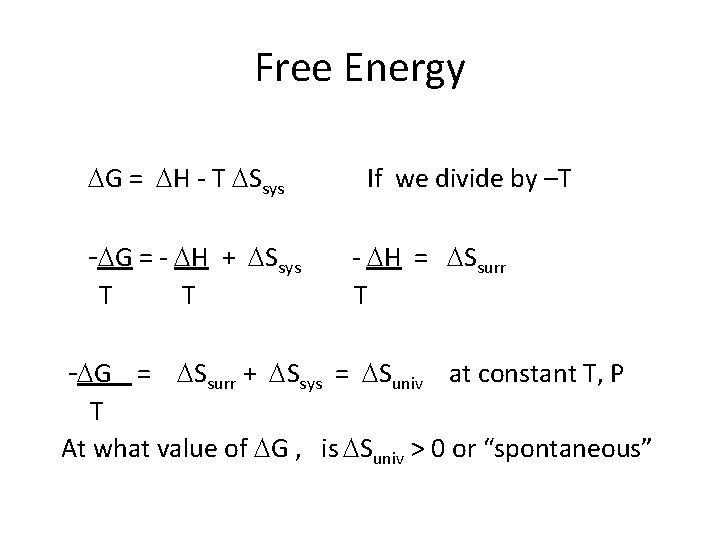
Free Energy G = H - T Ssys - G = - H + Ssys T T If we divide by –T - H = Ssurr T - G = Ssurr + Ssys = Suniv at constant T, P T At what value of G , is Suniv > 0 or “spontaneous”

