Thermochemistry Energy Transformations Calorimetry Calorimetry The measurement of




- Slides: 13

Thermochemistry Energy Transformations

Calorimetry • Calorimetry: The measurement of the heat into or out of a system for chemical and physical processes. • Based on the fact that the heat absorbed = the heat released • The device used to measure the absorption or release of heat is called a Calorimeter

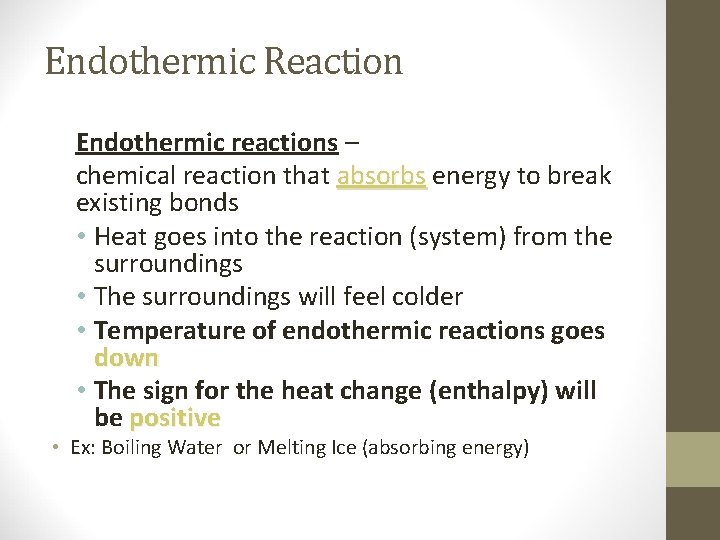
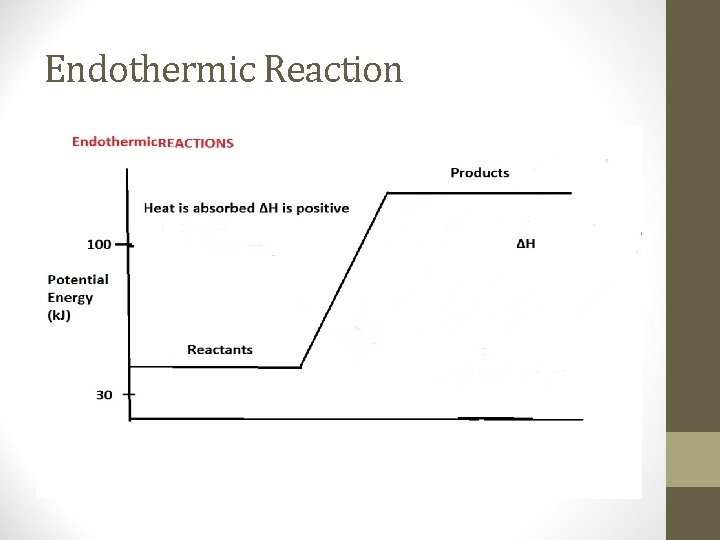
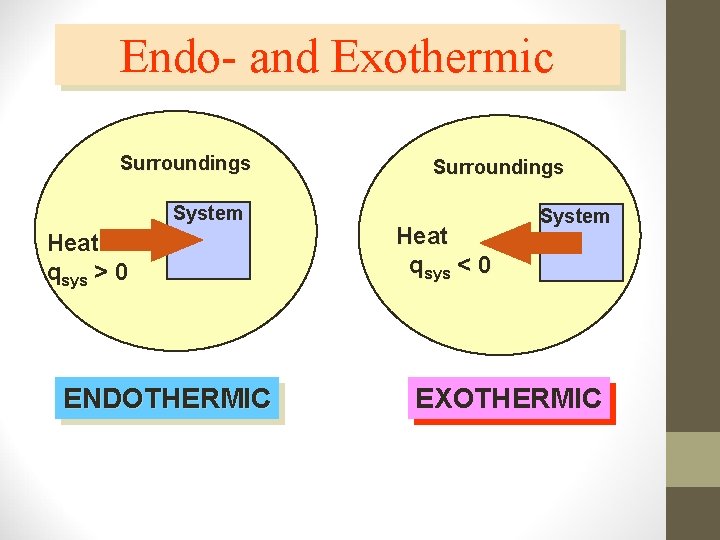
Endothermic Reaction Endothermic reactions – chemical reaction that absorbs energy to break existing bonds • Heat goes into the reaction (system) from the surroundings • The surroundings will feel colder • Temperature of endothermic reactions goes down • The sign for the heat change (enthalpy) will be positive • Ex: Boiling Water or Melting Ice (absorbing energy)

Endothermic Reaction

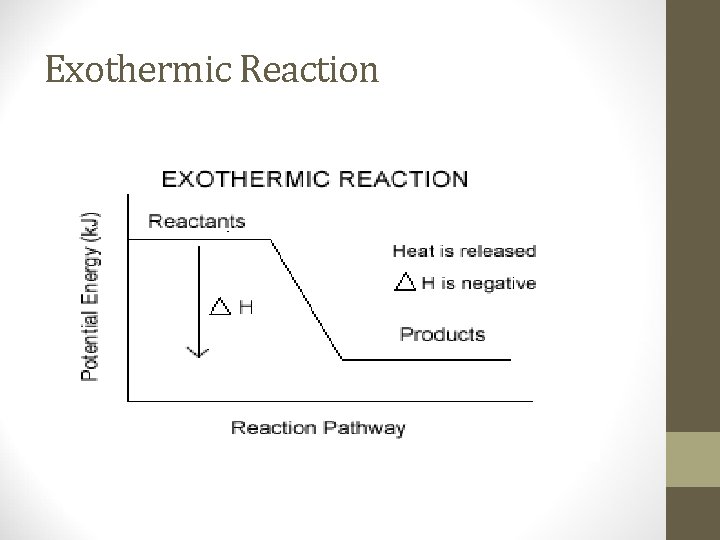
Exothermic Reactions • Exothermic reactions – chemical reaction in which energy is released • Heat goes out of the reaction (system) into the surroundings • The surroundings will feel hotter • Temperature of exothermic reactions goes up • The sign for the heat change(enthalpy) will be negative • EX: Condensation (gas to liquid) releasing energy!

Exothermic Reaction

Endo- and Exothermic Surroundings System Heat qsys > 0 ENDOTHERMIC Surroundings Heat qsys < 0 System EXOTHERMIC

Endothermic & Exothermic Reactions • Are the following reactions endothermic or exothermic? • CO + 3 H 2 CH 4 + H 2 O H= -206 k. J • I add magnesium metal to some hydrochloric acid. The temperature goes from 23 C to 27 C • I mix together some vinegar & baking soda. The temperature goes from 28 C to 23 C

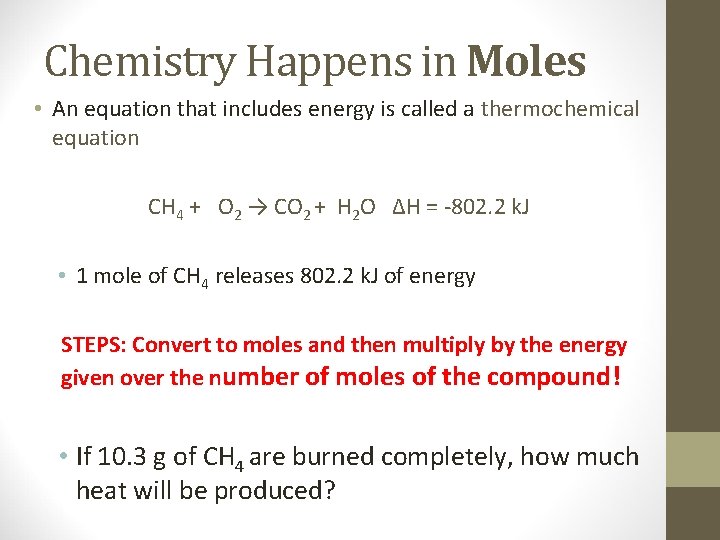
Chemistry Happens in Moles • An equation that includes energy is called a thermochemical equation CH 4 + O 2 → CO 2 + H 2 O ΔH = -802. 2 k. J • 1 mole of CH 4 releases 802. 2 k. J of energy STEPS: Convert to moles and then multiply by the energy given over the number of moles of the compound! • If 10. 3 g of CH 4 are burned completely, how much heat will be produced?

The Work

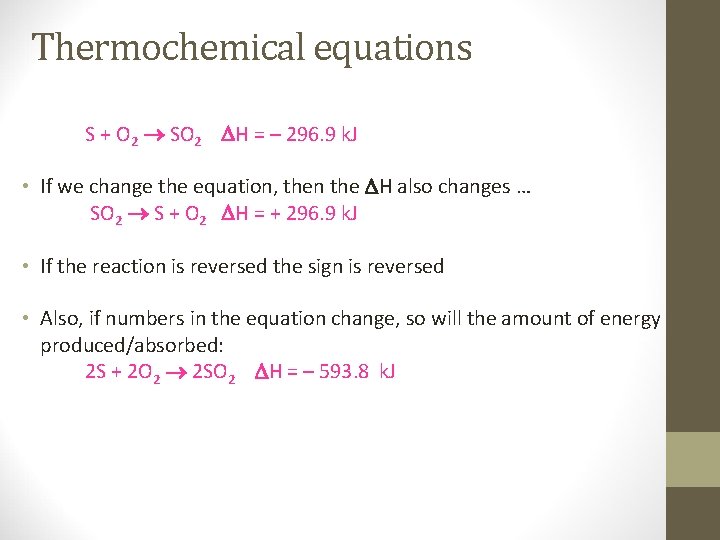
Thermochemical equations S + O 2 SO 2 H = – 296. 9 k. J • If we change the equation, then the H also changes … SO 2 S + O 2 H = + 296. 9 k. J • If the reaction is reversed the sign is reversed • Also, if numbers in the equation change, so will the amount of energy produced/absorbed: 2 S + 2 O 2 2 SO 2 H = – 593. 8 k. J

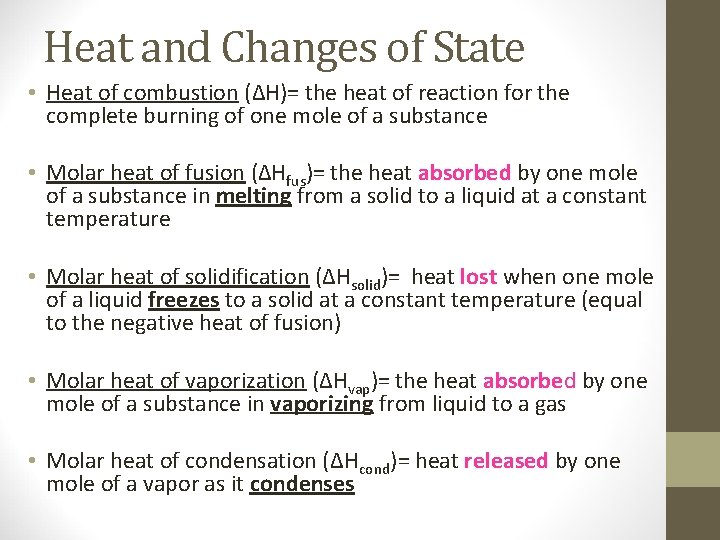
Heat and Changes of State • Heat of combustion (∆H)= the heat of reaction for the complete burning of one mole of a substance • Molar heat of fusion (∆Hfus)= the heat absorbed by one mole of a substance in melting from a solid to a liquid at a constant temperature • Molar heat of solidification (∆Hsolid)= heat lost when one mole of a liquid freezes to a solid at a constant temperature (equal to the negative heat of fusion) • Molar heat of vaporization (∆Hvap)= the heat absorbed by one mole of a substance in vaporizing from liquid to a gas • Molar heat of condensation (∆Hcond)= heat released by one mole of a vapor as it condenses

Example (Heat of combustion) • The standard heat of combustion (∆H°rxn) for glucose (C 6 H 12 O 6) is -2808 k. J/mol. If you eat and burn 71 g of glucose in one day, how much energy are you getting from the glucose? • C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O ΔH = -2808 k. J • Step one: convert g of glucose to moles • Step two: Use (∆H°rxn) to find amount of k. J gained
 Hess law constant heat summation
Hess law constant heat summation Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Digestive system energy transformation
Digestive system energy transformation Clock energy transformation
Clock energy transformation Energy transformations in photosynthesis
Energy transformations in photosynthesis Multiple energy transformations
Multiple energy transformations Graphic organizer with its energy transformation
Graphic organizer with its energy transformation 7 types of energy
7 types of energy Identify the energy transformations that occur in a guitar
Identify the energy transformations that occur in a guitar Energy transformations and conservation
Energy transformations and conservation 3 types of energy transformations
3 types of energy transformations