Thermal Expansion Contraction Changes of State What have

- Slides: 11

Thermal Expansion & Contraction Changes of State

What have we learned about so far? • Turn to the person beside you and tell them 3 things you learned last class. – We learned about… • Matter, Mass & Volume • Three states of matter (solid, liquid gas) • Particle model and the Kinetic Molecular Theory

Thermal Expansion and Contraction • Kinetic Energy (KE) is the energy of motion. • When you add energy to something, you increase the KE. • One way to add energy to an object is to add HEAT. • Adding heat makes particles: • A) move FASTER, • B) cover more area and • C) the material EXPANDS in volume! • This process is called…. Thermal Expansion

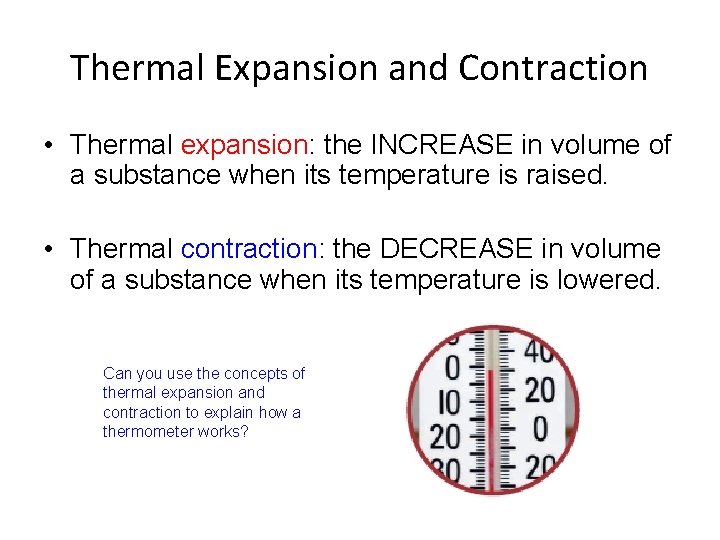
Thermal Expansion and Contraction • Thermal expansion: the INCREASE in volume of a substance when its temperature is raised. • Thermal contraction: the DECREASE in volume of a substance when its temperature is lowered. Can you use the concepts of thermal expansion and contraction to explain how a thermometer works?

Thermal Expansion and Contraction • In thermal contraction particles slow down and take up less space. • There is one exception to thermal contraction can you guess what it is? – WATER! When water cools and freezes it expands!

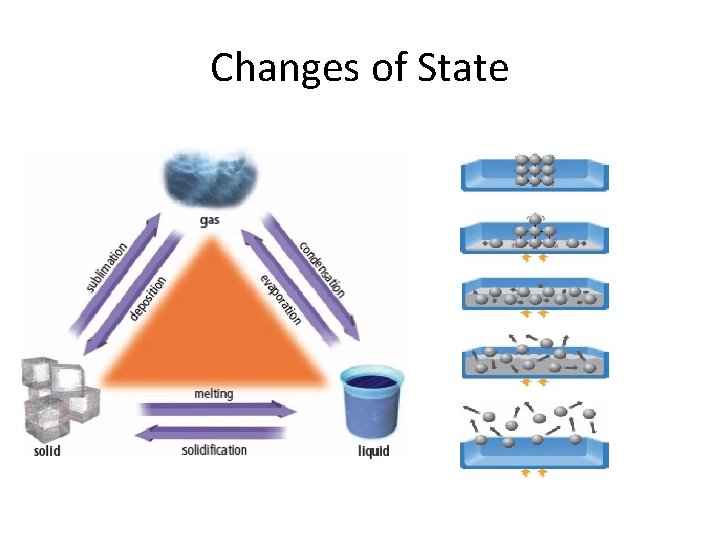
Changes of State

Changes of State • Melting Point: the temperature at which solid turns to liquid. • Boiling Point: the temperature at which liquid turns into gas.

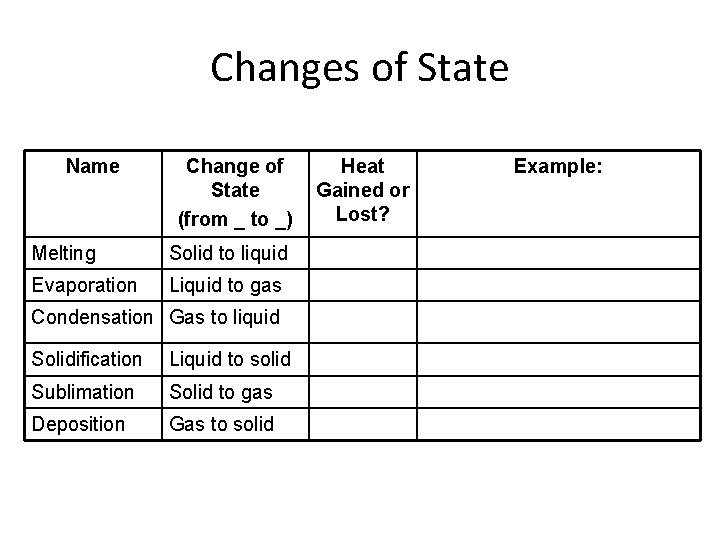
Changes of State Name Change of State (from _ to _) Melting Solid to liquid Evaporation Liquid to gas Condensation Gas to liquid Solidification Liquid to solid Sublimation Solid to gas Deposition Gas to solid Heat Gained or Lost? Example:

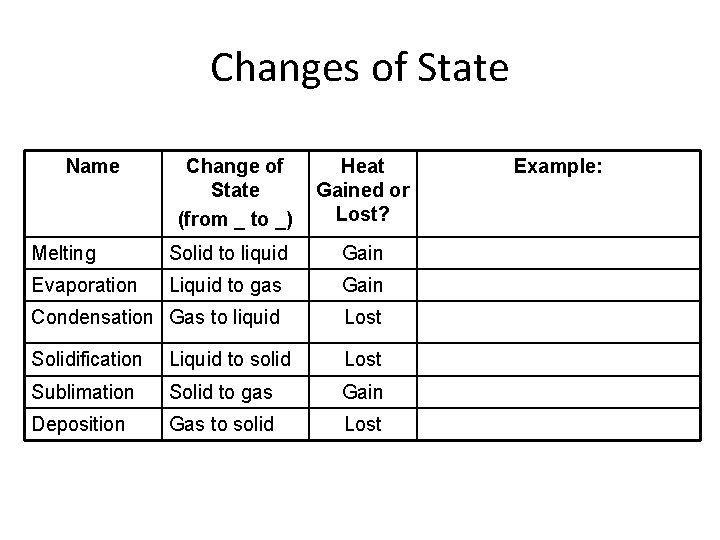
Changes of State Name Change of State (from _ to _) Heat Gained or Lost? Melting Solid to liquid Gain Evaporation Liquid to gas Gain Condensation Gas to liquid Lost Solidification Liquid to solid Lost Sublimation Solid to gas Gain Deposition Gas to solid Lost Example:

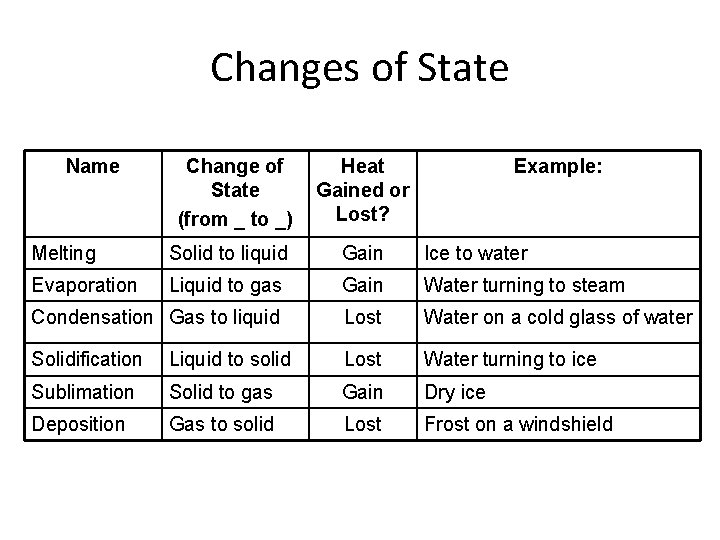
Changes of State Name Change of State (from _ to _) Heat Gained or Lost? Example: Melting Solid to liquid Gain Ice to water Evaporation Liquid to gas Gain Water turning to steam Condensation Gas to liquid Lost Water on a cold glass of water Solidification Liquid to solid Lost Water turning to ice Sublimation Solid to gas Gain Dry ice Deposition Gas to solid Lost Frost on a windshield

Thermal Expansion & Contraction
 Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Smooth muslce
Smooth muslce Horizontal contraction/expansion matrix
Horizontal contraction/expansion matrix Expansion and contraction weathering
Expansion and contraction weathering Expansion and contraction
Expansion and contraction Thermostatic expansion valves respond to changes in
Thermostatic expansion valves respond to changes in Thermal expansion examples
Thermal expansion examples Thermal expansion in liquids
Thermal expansion in liquids Thermometer and thermal expansion
Thermometer and thermal expansion Thermometer thermal expansion
Thermometer thermal expansion Why does water behave in this unusual fashion?
Why does water behave in this unusual fashion? Thermal expansion notes
Thermal expansion notes