Reading Quiz Temperature 1 All matter is made















- Slides: 30

Reading Quiz - Temperature 1. All matter is made of ___ 1. Atoms. ___ 2. Combinations of earth, wind, fire and water. ___ 3. Identical particles. ___ 4. Cells.

2. Which of the following is a temperature scale? ___ 1. Celsius. ___ 2. Fahrenheit ___ 3. Kelvin. ___ 4. All of the above.

3. Most materials will ___ 1. not be affected ___ 2. expand ___ 3. contract when heated.

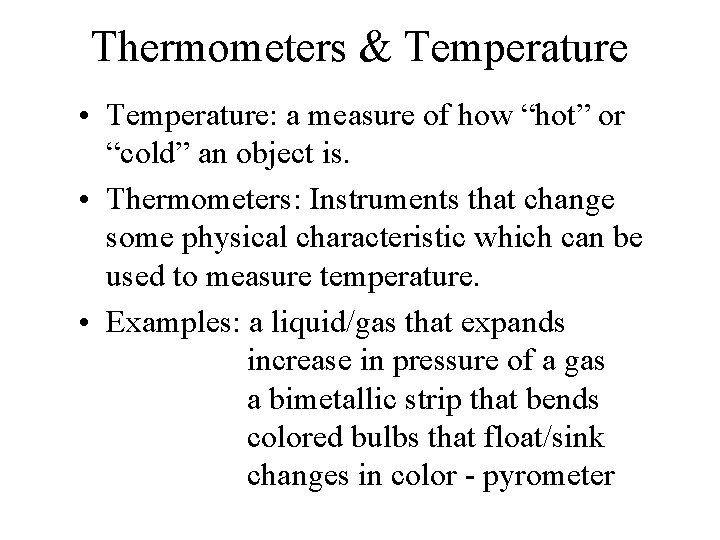
Thermometers & Temperature • Temperature: a measure of how “hot” or “cold” an object is. • Thermometers: Instruments that change some physical characteristic which can be used to measure temperature. • Examples: a liquid/gas that expands increase in pressure of a gas a bimetallic strip that bends colored bulbs that float/sink changes in color - pyrometer

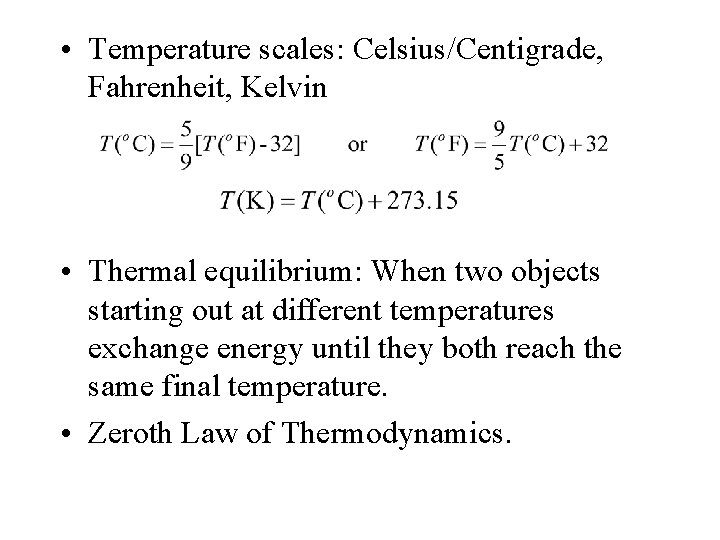
• Temperature scales: Celsius/Centigrade, Fahrenheit, Kelvin • Thermal equilibrium: When two objects starting out at different temperatures exchange energy until they both reach the same final temperature. • Zeroth Law of Thermodynamics.

Conceptual Questions 1) What is the problem with 0°C = 32°F = 273. 15 K? ____ a) this is mathematically incorrect ____ b) this is mathematically correct ____ c) mixes units ____ d) there is no problem

2) Three different types of thermometers are used to read the temperature of a warm glass milk. The readings are ____ a) the same for all three thermometers ____ b) the three read slightly different ____ c) the three read very different temperatures

Quantitative Questions 1) At what temperature is the Celsius scale the same as the Fahrenheit scale? 2) The body temperature of a healthy human is 98. 6°F. Express this in degrees Celsius.

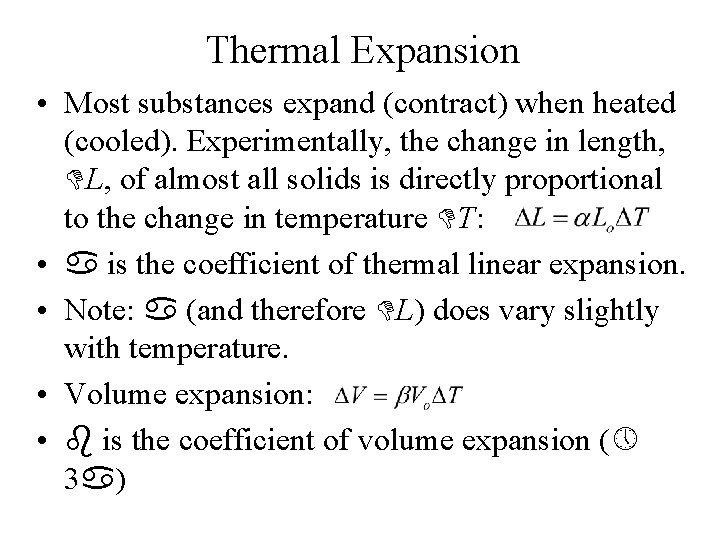
Thermal Expansion • Most substances expand (contract) when heated (cooled). Experimentally, the change in length, L, of almost all solids is directly proportional to the change in temperature T: • is the coefficient of thermal linear expansion. • Note: (and therefore L) does vary slightly with temperature. • Volume expansion: • is the coefficient of volume expansion ( 3 )

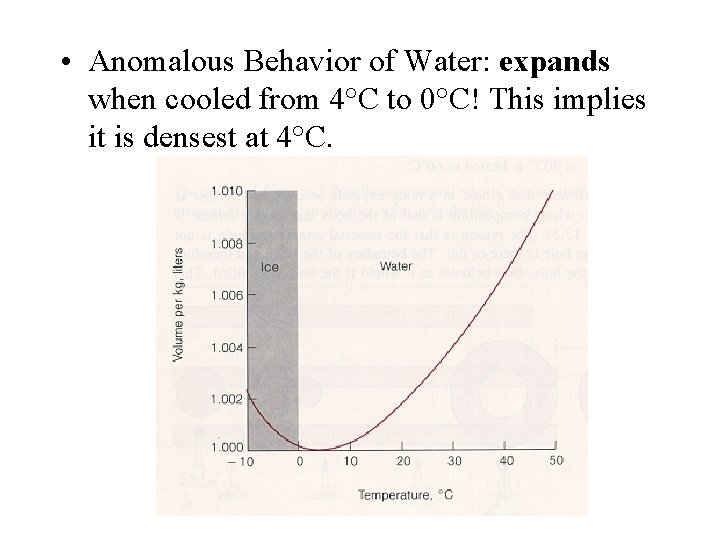
• Anomalous Behavior of Water: expands when cooled from 4°C to 0°C! This implies it is densest at 4°C.

• Great importance to the survival of aquatic life during cold winters! • It expands even more as it freezes: evidence - ice floats in water and pipes break when water in them freezes.

Conceptual Questions 1) The height of a column of mercury in a thermometer when placed in hot water ____ a) rises immediately ____ b) descends slightly and then rises ____ c) rises and then descends slightly ____ d) descends immediately

2) Rubber has a negative coefficient of linear expansion. What happens to the size of a piece of rubber as it is warmed? ____ a) it expands ____ b) it remains the same ____ c) it contracts ____ d) it expands non-uniformly ____ e) it contracts non-uniformly

3) A bimetallic strip, consisting of metal G on the top and metal H on the bottom, is rigidly attached to a wall at the left. The coefficient of linear thermal expansion for metal G is greater than that of metal H. If the strip is uniformly heated, it will ____ a) curve upward. ____ b) curve downward. ____ c) remain horizontal, but get longer. ____ d) bend in the middle.

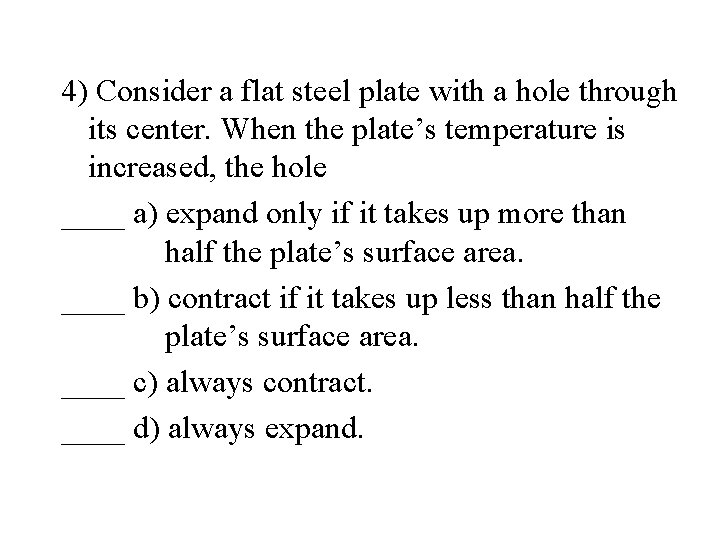
4) Consider a flat steel plate with a hole through its center. When the plate’s temperature is increased, the hole ____ a) expand only if it takes up more than half the plate’s surface area. ____ b) contract if it takes up less than half the plate’s surface area. ____ c) always contract. ____ d) always expand.

5) The surface water temperature on a large, deep lake is 3°C. A sensitive temperature probe is lowered several meters into the lake. What temperature will the probe read? ____ a) A temperature warmer than 3°C. ____ b) A temperature less than 3°C. ____ c) A temperature equal to 3°C. ____ d) There is not enough information to determine the answer.

Quantitative Problems 1) The steel bed of a suspension bridge is 200 m long at 20°C. If the extremes of temperature to which it might be exposed are -30°C to +40°C, how much will it contract and expand? 2) Vodka that is “ 100 proof” is a mixture of half ethyl alcohol and half water (by volume). How much profit per liter will a merchant make if he buys vodka at $10 per liter at 0°C and sells it at $10 per liter at 25°C?

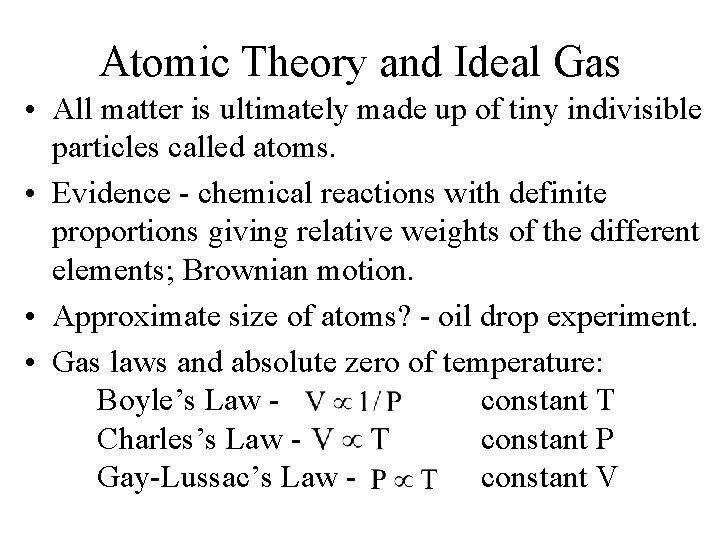
Atomic Theory and Ideal Gas • All matter is ultimately made up of tiny indivisible particles called atoms. • Evidence - chemical reactions with definite proportions giving relative weights of the different elements; Brownian motion. • Approximate size of atoms? - oil drop experiment. • Gas laws and absolute zero of temperature: Boyle’s Law constant T Charles’s Law constant P Gay-Lussac’s Law constant V

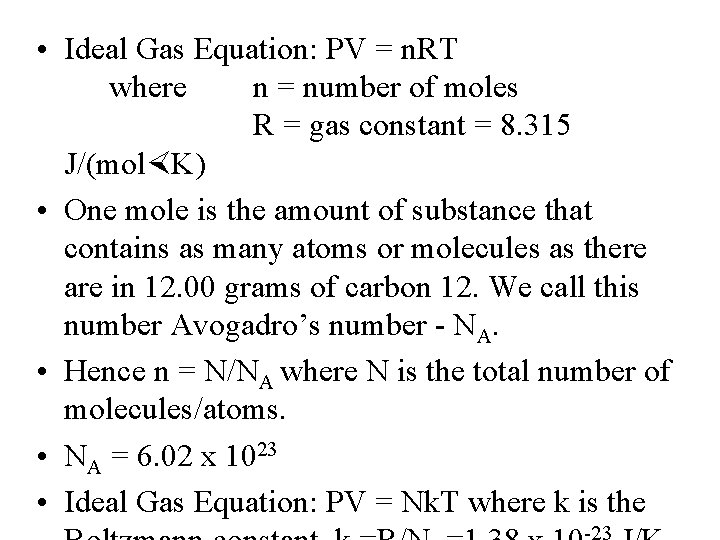
• Ideal Gas Equation: PV = n. RT where n = number of moles R = gas constant = 8. 315 J/(mol K) • One mole is the amount of substance that contains as many atoms or molecules as there are in 12. 00 grams of carbon 12. We call this number Avogadro’s number - NA. • Hence n = N/NA where N is the total number of molecules/atoms. • NA = 6. 02 x 1023 • Ideal Gas Equation: PV = Nk. T where k is the

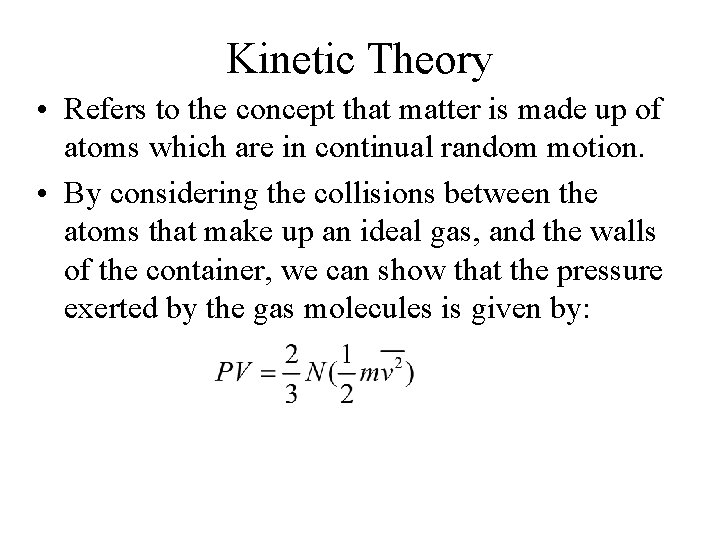
Kinetic Theory • Refers to the concept that matter is made up of atoms which are in continual random motion. • By considering the collisions between the atoms that make up an ideal gas, and the walls of the container, we can show that the pressure exerted by the gas molecules is given by:

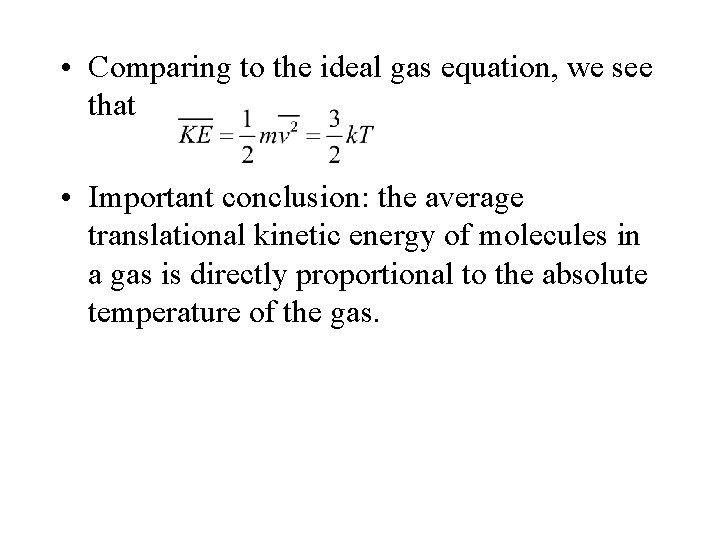
• Comparing to the ideal gas equation, we see that • Important conclusion: the average translational kinetic energy of molecules in a gas is directly proportional to the absolute temperature of the gas.

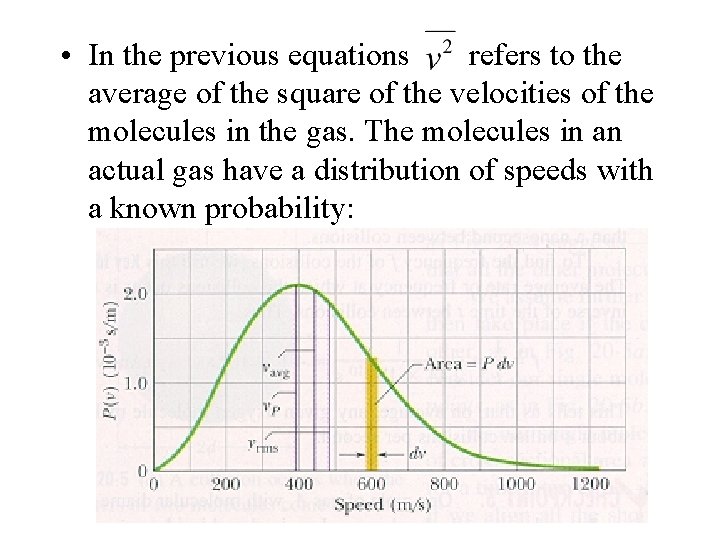
• In the previous equations refers to the average of the square of the velocities of the molecules in the gas. The molecules in an actual gas have a distribution of speeds with a known probability:

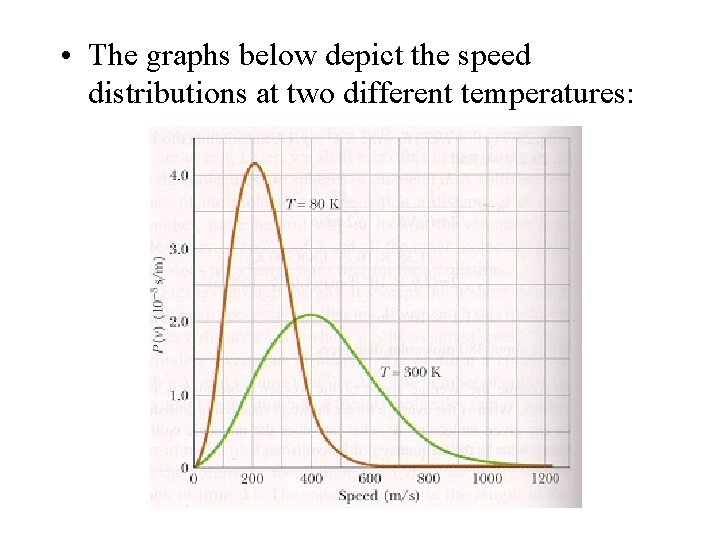
• The graphs below depict the speed distributions at two different temperatures:

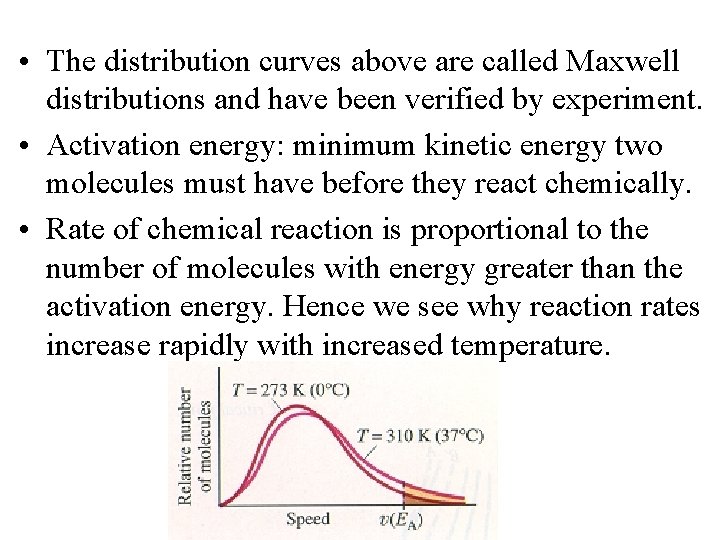
• The distribution curves above are called Maxwell distributions and have been verified by experiment. • Activation energy: minimum kinetic energy two molecules must have before they react chemically. • Rate of chemical reaction is proportional to the number of molecules with energy greater than the activation energy. Hence we see why reaction rates increase rapidly with increased temperature.

Conceptual Question 1) According to the ideal gas Law, PV = constant for a given temperature. As a result, an increase in volume corresponds to a decrease in pressure. This happens because the molecules ____ a) collide with each other more frequently. ____ b) move slower on the average. ____ c) strike the container wall less often. ____ d) transfer less energy to the walls of the container each time they strike it.

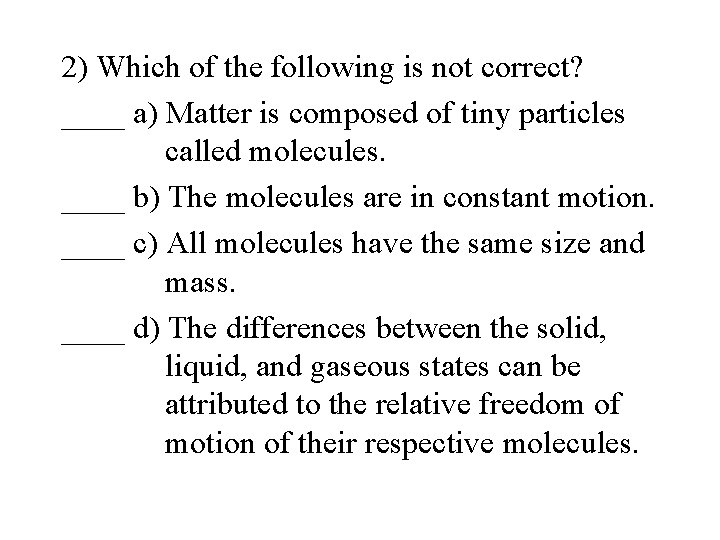
2) Which of the following is not correct? ____ a) Matter is composed of tiny particles called molecules. ____ b) The molecules are in constant motion. ____ c) All molecules have the same size and mass. ____ d) The differences between the solid, liquid, and gaseous states can be attributed to the relative freedom of motion of their respective molecules.

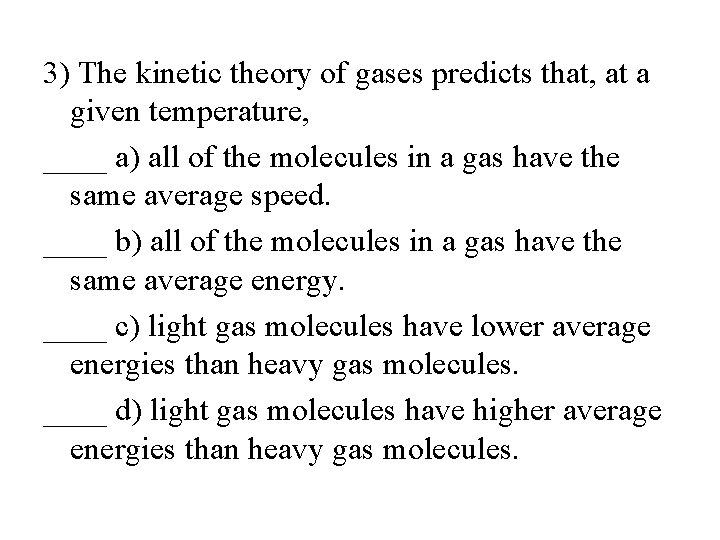
3) The kinetic theory of gases predicts that, at a given temperature, ____ a) all of the molecules in a gas have the same average speed. ____ b) all of the molecules in a gas have the same average energy. ____ c) light gas molecules have lower average energies than heavy gas molecules. ____ d) light gas molecules have higher average energies than heavy gas molecules.

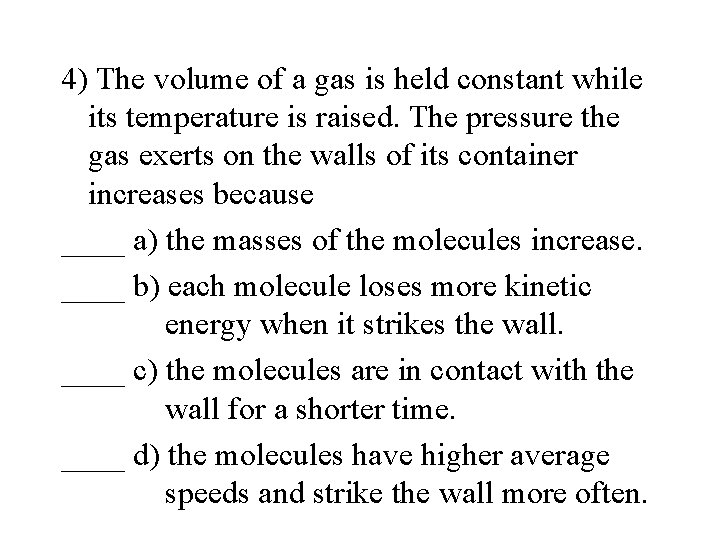
4) The volume of a gas is held constant while its temperature is raised. The pressure the gas exerts on the walls of its container increases because ____ a) the masses of the molecules increase. ____ b) each molecule loses more kinetic energy when it strikes the wall. ____ c) the molecules are in contact with the wall for a shorter time. ____ d) the molecules have higher average speeds and strike the wall more often.

5) The temperature of a gas is held constant while its volume is reduced. The pressure the gas exerts on the walls of its container increases because its molecules ____ a) strike the container walls more often. ____ b) strike the container walls with higher speeds. ____ c) strike the container walls with greater force. ____ d) have more energy.

Quantitative Problems 1) What is the average translational kinetic energy of molecules in a gas at 37°C? 2) Since refers to the average of the square of the velocity, the square-root of this quantity is called the root-mean-square speed or. If a gas is at 0°C, to what temperature must it be raised to double the rms speed of its molecules? 3) Why does the moon have no atmosphere?