SLG SOLID NORMALLY CAUSES A TEMPERATURE CHANGE IN












- Slides: 18

SLG

SOLID NORMALLY CAUSES A TEMPERATURE CHANGE IN THE SOLID. WHAT HAPPENS TO THERMAL ENERGY AND TEMPERATURE AS A SOLID MELTS? Temperature remains constant during melting; additional thermal energy is being used to break solid particles apart

THERMAL ENERGY IS ADDED TO WATER TO TURN IT INTO STEAM. EXPLAIN THIS EVENT ACCORDING TO THE KINETIC THEORY OF MATTER. Water molecules are constantly moving but are held together by attractions between molecules; as thermal energy is added, molecules move more quickly and can overcome attraction and escape as steam.

A COLD GLASS WILL COLLECT MOISTURE ON A HOT, HUMID DAY. EXPLAIN THIS EVENT ACCORDING TO THE KINETIC THEORY OF MATTER. Water vapor in air transfers thermal energy to the cooler glass. This slows the molecules, and condensation occurs.

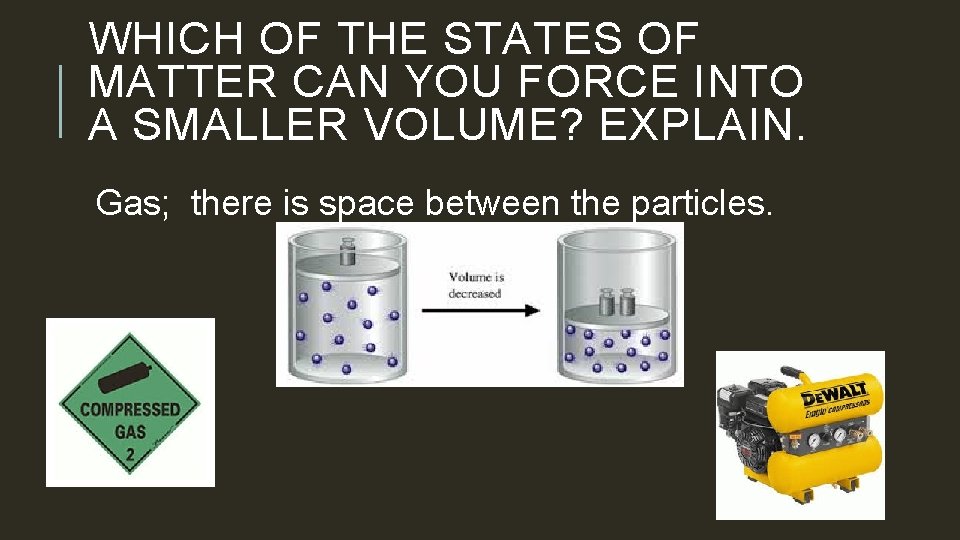
WHICH OF THE STATES OF MATTER CAN YOU FORCE INTO A SMALLER VOLUME? EXPLAIN. Gas; there is space between the particles.

EXPLAIN THERMAL EXPANSION OF A SOLID USING THE KINETIC THEORY OF MATTER. As thermal energy is added to the solid, the particles gain kinetic energy and move faster, causing the particles to take up more space

EXPLAIN WHAT HAPPENS AT THE SURFACE OF A LIQUID ACCORDING TO THE KINETIC THEORY AS THERMAL ENERGY IS ADDED TO THE LIQUID. Liquid molecules are free to move and have a range of kinetic energies. They will gain kinetic energy from added thermal energy, and some particles can escape the surface if they have enough energy.

EXPLAIN THE PURPOSE OF AN EXPANSION JOINT ON A BRIDGE. As the bridge heats in the summer, it becomes longer; in the winter, it becomes shorter.

WHAT WILL HAPPEN TO THE SIZE OF A BALLOON WHEN IT IS PLACED IN A FREEZER? EXPLAIN. It shrinks; as the temperature of air decreases, the volume of the air decreases as particles move closer together.

LIST THE STATES OF MATTER AND EXPLAIN THEIR DIFFERENCES USING THE KINETIC THEORY OF MATTER. solid—particles closest together, not free to move around; liquid—particles close together but free to move (flow); gas—particles farthest apart, free to move in any direction

WHAT STRANGE PROPERTY OF WATER DOES A GLASS OF ICE WATER ILLUSTRATE? This illustrates the expansion of water as it goes from a liquid to a solid. Because solid water (ice) is less dense than liquid water, it floats.

IN TERMS OF THERMAL EXPANSION, WHY IS WATER UNUSUAL? Water expands when it goes from the liquid to the solid state.

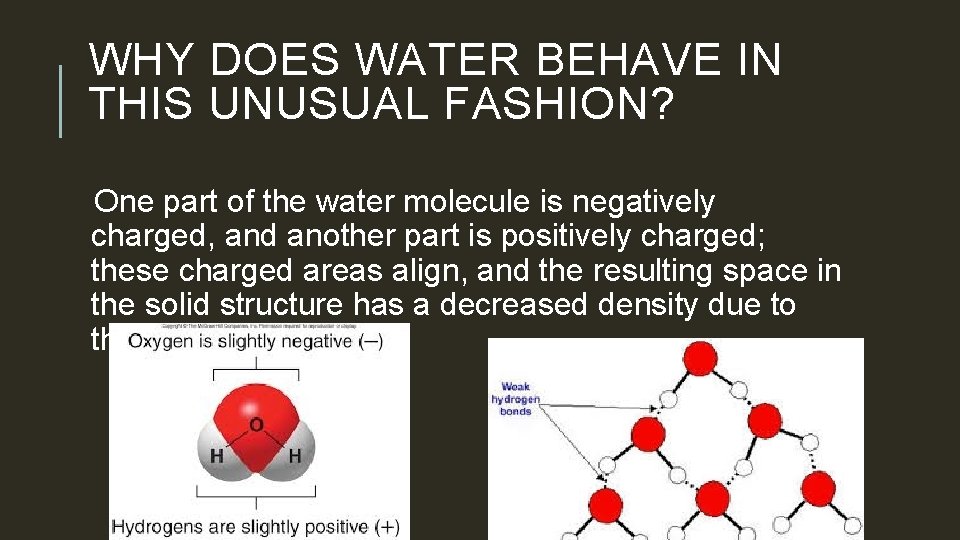
WHY DOES WATER BEHAVE IN THIS UNUSUAL FASHION? One part of the water molecule is negatively charged, and another part is positively charged; these charged areas align, and the resulting space in the solid structure has a decreased density due to the increased volume.

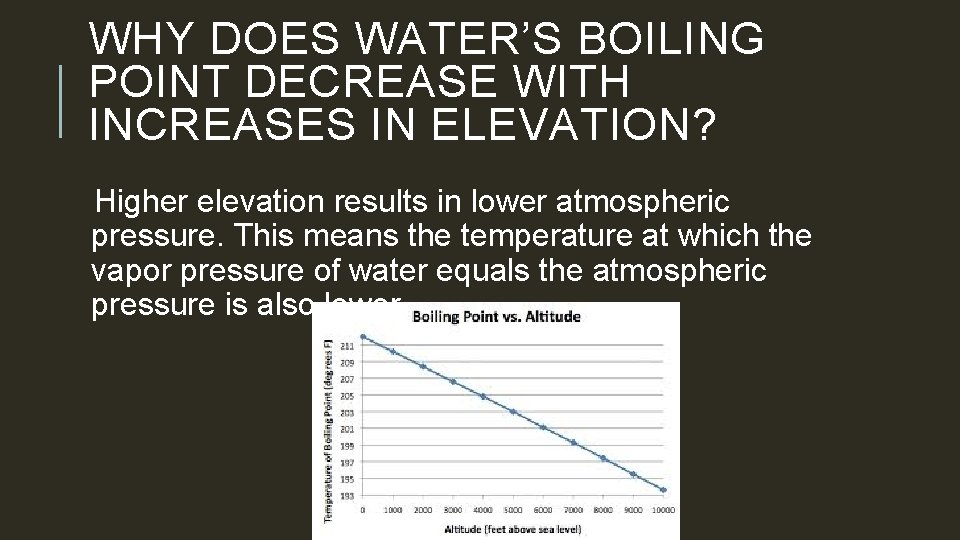
WHY DOES WATER’S BOILING POINT DECREASE WITH INCREASES IN ELEVATION? Higher elevation results in lower atmospheric pressure. This means the temperature at which the vapor pressure of water equals the atmospheric pressure is also lower.

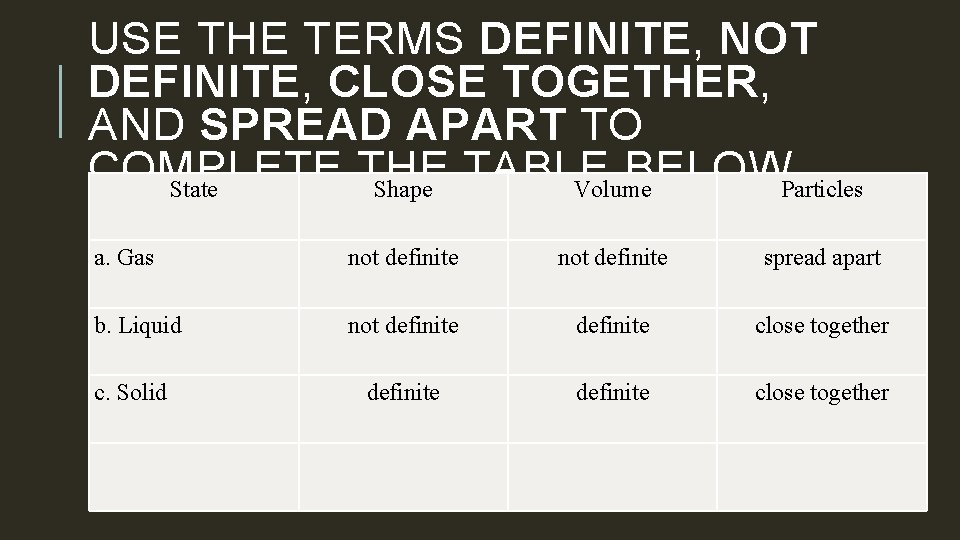
USE THE TERMS DEFINITE, NOT DEFINITE, CLOSE TOGETHER, AND SPREAD APART TO COMPLETE THE TABLE BELOW. State Shape Volume Particles a. Gas not definite spread apart b. Liquid not definite close together c. Solid

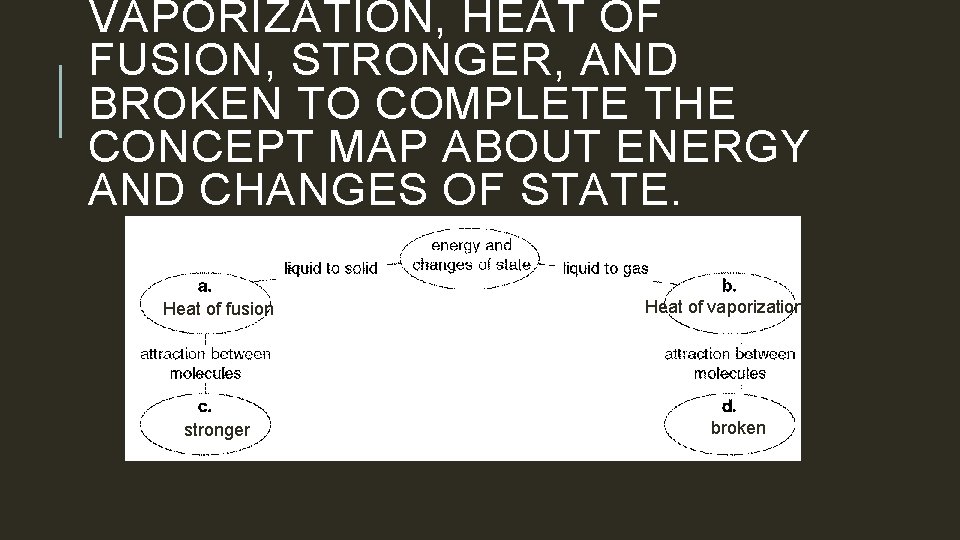
VAPORIZATION, HEAT OF FUSION, STRONGER, AND BROKEN TO COMPLETE THE CONCEPT MAP ABOUT ENERGY AND CHANGES OF STATE. Heat of fusion stronger Heat of vaporization broken

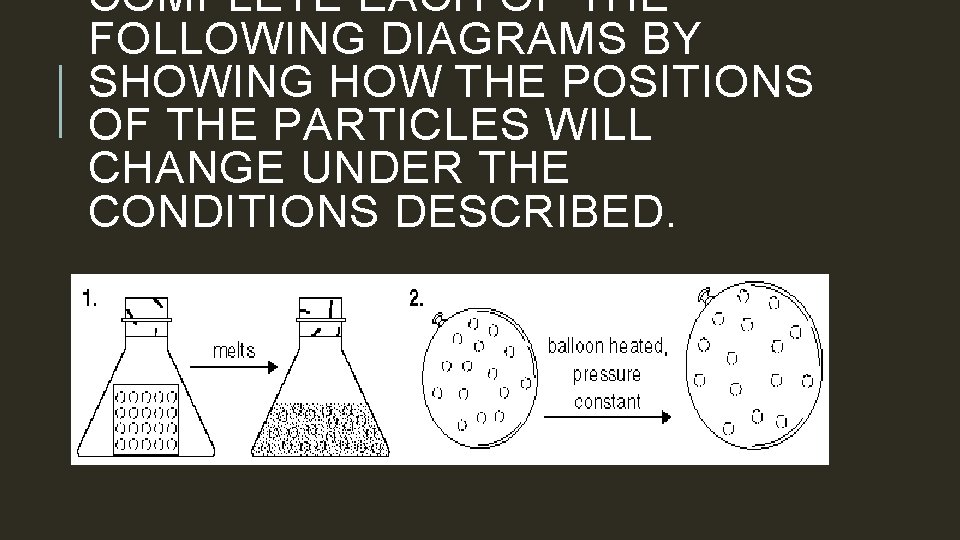
COMPLETE EACH OF THE FOLLOWING DIAGRAMS BY SHOWING HOW THE POSITIONS OF THE PARTICLES WILL CHANGE UNDER THE CONDITIONS DESCRIBED.

WHERE MIGHT YOU FIND PLASMA? Sun, stars, nuclear reactors, and lightning.