The Effects of Necrotizing Enterocolitis on Cytoskeletal Pathways

- Slides: 2

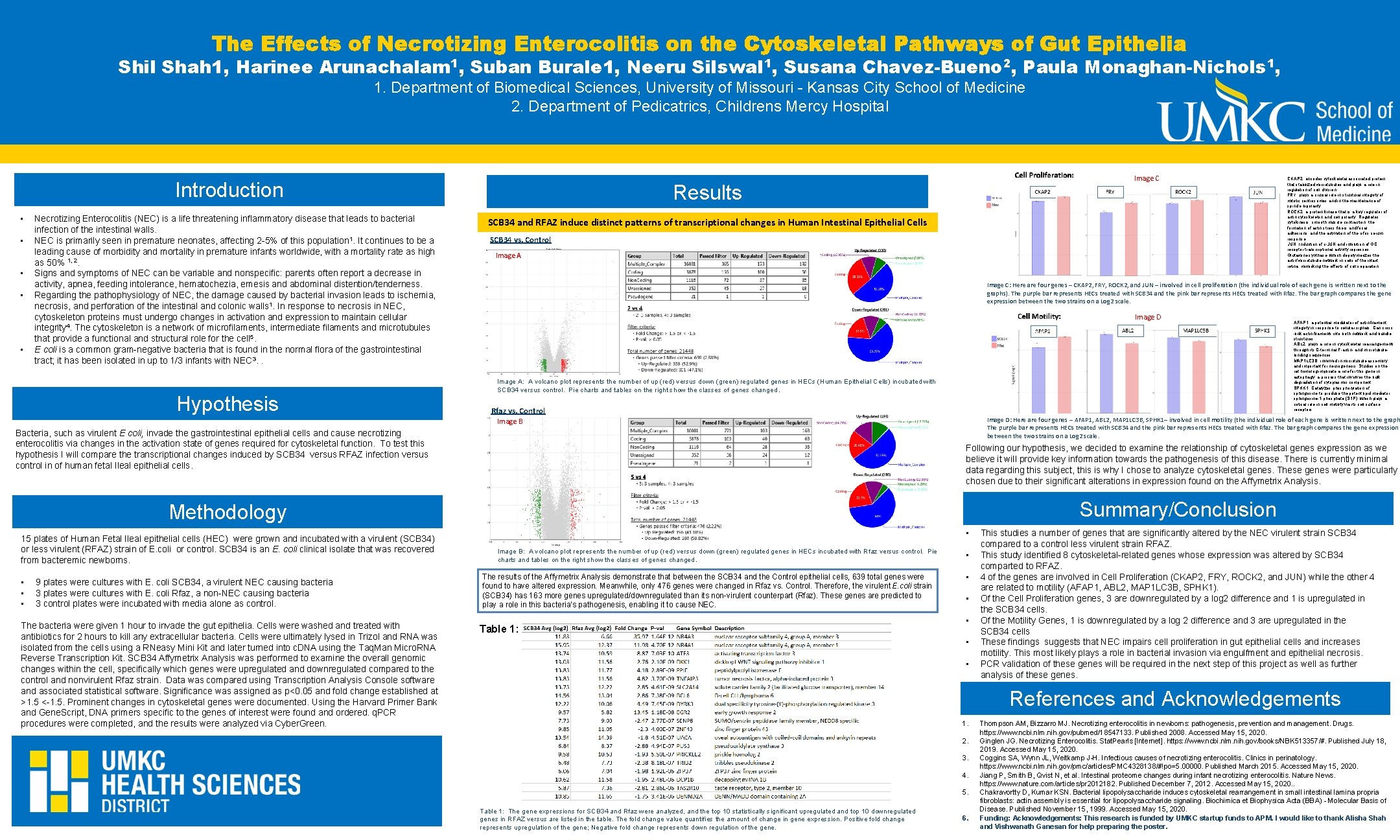
The Effects of Necrotizing Enterocolitis on Cytoskeletal Pathways of Gut Epithelia Title ofthe Poster Shil Shah 1, Harinee Arunachalam 1, Suban Burale 1, Neeru Silswal Authors names 1, Susana Chavez-Bueno 2, Paula Monaghan-Nichols 1, 1. Department of Biomedical Sciences, University of Missouri - Kansas City School of Medicine Affiliations 2. Department of Pedicatrics, Childrens Mercy Hospital Introduction • • • Necrotizing Enterocolitis (NEC) is a life threatening inflammatory disease that leads to bacterial infection of the intestinal walls. NEC is primarily seen in premature neonates, affecting 2 -5% of this population 1. It continues to be a leading cause of morbidity and mortality in premature infants worldwide, with a mortality rate as high as 50% 1, 2. Signs and symptoms of NEC can be variable and nonspecific: parents often report a decrease in activity, apnea, feeding intolerance, hematochezia, emesis and abdominal distention/tenderness. Regarding the pathophysiology of NEC, the damage caused by bacterial invasion leads to ischemia, necrosis, and perforation of the intestinal and colonic walls 1. In response to necrosis in NEC, cytoskeleton proteins must undergo changes in activation and expression to maintain cellular integrity 4. The cytoskeleton is a network of microfilaments, intermediate filaments and microtubules that provide a functional and structural role for the cell 5. E coli is a common gram-negative bacteria that is found in the normal flora of the gastrointestinal tract; it has been isolated in up to 1/3 infants with NEC 3. . Hypothesis Results SCB 34 and RFAZ induce distinct patterns of transcriptional changes in Human Intestinal Epithelial Cells SCB 34 vs. Control Image A Rfaz vs. Control Bacteria, such as virulent E coli, invade the gastrointestinal epithelial cells and cause necrotizing enterocolitis via changes in the activation state of genes required for cytoskeletal function. To test this hypothesis I will compare the transcriptional changes induced by SCB 34 versus RFAZ infection versus control in of human fetal Ileal epithelial cells. 15 plates of Human Fetal Ileal epithelial cells (HEC) were grown and incubated with a virulent (SCB 34) or less virulent (RFAZ) strain of E. coli or control. SCB 34 is an E. coli clinical isolate that was recovered from bacteremic newborns. • • • 9 plates were cultures with E. coli SCB 34, a virulent NEC causing bacteria 3 plates were cultures with E. coli Rfaz, a non-NEC causing bacteria 3 control plates were incubated with media alone as control. The bacteria were given 1 hour to invade the gut epithelia. Cells were washed and treated with antibiotics for 2 hours to kill any extracellular bacteria. Cells were ultimately lysed in Trizol and RNA was isolated from the cells using a RNeasy Mini Kit and later turned into c. DNA using the Taq. Man Micro. RNA Reverse Transcription Kit. SCB 34 Affymetrix Analysis was performed to examine the overall genomic changes within the cell, specifically which genes were upregulated and downregulated compared to the control and nonvirulent Rfaz strain. Data was compared using Transcription Analysis Console software and associated statistical software. Significance was assigned as p<0. 05 and fold change established at >1. 5 <-1. 5. Prominent changes in cytoskeletal genes were documented. Using the Harvard Primer Bank and Gene. Script, DNA primers specific to the genes of interest were found and ordered. q. PCR procedures were completed, and the results were analyzed via Cyber. Green. Image D The results of the Affymetrix Analysis demonstrate that between the SCB 34 and the Control epithelial cells, 639 total genes were found to have altered expression. Meanwhile, only 476 genes were changed in Rfaz vs. Control. Therefore, the virulent E. coli strain (SCB 34) has 163 more genes upregulated/downregulated than its non-virulent counterpart (Rfaz). These genes are predicted to play a role in this bacteria’s pathogenesis, enabling it to cause NEC. • • • This studies a number of genes that are significantly altered by the NEC virulent strain SCB 34 compared to a control less virulent strain RFAZ. This study identified 8 cytoskeletal-related genes whose expression was altered by SCB 34 comparted to RFAZ. 4 of the genes are involved in Cell Proliferation (CKAP 2, FRY, ROCK 2, and JUN) while the other 4 are related to motility (AFAP 1, ABL 2, MAP 1 LC 3 B, SPHK 1). Of the Cell Proliferation genes, 3 are downregulated by a log 2 difference and 1 is upregulated in the SCB 34 cells. Of the Motility Genes, 1 is downregulated by a log 2 difference and 3 are upregulated in the SCB 34 cells These findings suggests that NEC impairs cell proliferation in gut epithelial cells and increases motility. This most likely plays a role in bacterial invasion via engulfment and epithelial necrosis. PCR validation of these genes will be required in the next step of this project as well as further analysis of these genes. References and Acknowledgements 1. 2. 3. 4. 5. Table 1: The gene expressions for SCB 34 and Rfaz were analyzed, and the top 10 statistically significant upregulated and top 10 downregulated genes in RFAZ versus are listed in the table. The fold change value quantifies the amount of change in gene expression. Positive fold change represents upregulation of the gene; Negative fold change represents down regulation of the gene. Image D: Here are four genes – AFAP 1, ABL 2, MAP 1 LC 3 B, SPHK 1– involved in cell motility (the individual role of each gene is written next to the graph The purple bar represents HECs treated with SCB 34 and the pink bar represents HECs treated with Rfaz. The bar graph compares the gene expression between the two strains on a Log 2 scale. Summary/Conclusion • AFAP 1: a potential modulator of actin filament integrity in response to cellular signals. Can cross -link actin filaments into both network and bundle structures ABL 2: plays a role in cytoskeletal rearrangements through its C-terminal F-actin- and microtubulebinding sequences MAP 1 LC 3 B: involved in microtubule assembly and important for neurogenesis. Studies on the rat homolog implicate a role for this gene in autophagy, a process that involves the bulk degradation of cytoplasmic component SPHK 1: Catalyzes phosphorylation of sphingosine to produce the potent lipid mediator sphingosine-1 -phosphate (S 1 P), which plays a critical role in cell motility via its cell surface receptors. Following our hypothesis, we decided to examine the relationship of cytoskeletal genes expression as we believe it will provide key information towards the pathogenesis of this disease. There is currently minimal data regarding this subject, this is why I chose to analyze cytoskeletal genes. These genes were particularly chosen due to their significant alterations in expression found on the Affymetrix Analysis. • Image B: A volcano plot represents the number of up (red) versus down (green) regulated genes in HECs incubated with Rfaz versus control. Pie charts and tables on the right show the classes of genes changed. Table 1: CKAP 2: encodes cytoskeletal associated protein that stabilized microtubules and plays a role in regulation of cell division. FRY: plays a crucial role in structural integrity of mitotic centrosomes and in the maintenance of spindle bipolarity ROCK 2: a protein kinase that is a key regulator of actin cytoskeleton and cell polarity. Regulates cytokinesis, smooth muscle contraction, the formation of actin stress fibers and focal adhesions, and the activation of the c-fos serum response JUN: Induction of c-JUN and inhibition of GC receptor transcriptional activity represses Glutamine synthase, which depolymerizes the actin/microtubule network in cells of the intact retina, mimicking the effects of cell separation Image C: Here are four genes – CKAP 2, FRY, ROCK 2, and JUN – involved in cell proliferation (the individual role of each gene is written next to the graphs). The purple bar represents HECs treated with SCB 34 and the pink bar represents HECs treated with Rfaz. The bar graph compares the gene expression between the two strains on a Log 2 scale. Image A: A volcano plot represents the number of up (red) versus down (green) regulated genes in HECs (Human Epithelial Cells) incubated with SCB 34 versus control. Pie charts and tables on the right show the classes of genes changed. Image B Methodology Image C 6. Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs. https: //www. ncbi. nlm. nih. gov/pubmed/18547133. Published 2008. Accessed May 15, 2020. Ginglen JG. Necrotizing Enterocolitis. Stat. Pearls [Internet]. https: //www. ncbi. nlm. nih. gov/books/NBK 513357/#. Published July 18, 2019. Accessed May 15, 2020. Coggins SA, Wynn JL, Weitkamp J-H. Infectious causes of necrotizing enterocolitis. Clinics in perinatology. https: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 4328138/#!po=5. 00000. Published March 2015. Accessed May 15, 2020. Jiang P, Smith B, Qvist N, et al. Intestinal proteome changes during infant necrotizing enterocolitis. Nature News. https: //www. nature. com/articles/pr 2012182. Published December 7, 2012. Accessed May 15, 2020. . Chakravortty D, Kumar KSN. Bacterial lipopolysaccharide induces cytoskeletal rearrangement in small intestinal lamina propria fibroblasts: actin assembly is essential for lipopolysaccharide signaling. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. Published November 15, 1999. Accessed May 15, 2020. Funding: Acknowledgements: This research is funded by UMKC startup funds to APM. I would like to thank Alisha Shah and Vishwanath Ganesan for help preparing the poster.

Title of Poster Authors names Instructions Affiliations • Please do not change the font size, color, or background color of the template. You must use this template. • You can change the section headings or delete them in order to best present your research. For example, you may decide to delete the summary section. • You can change the size of each section box for pictures/graphs, etc. However, all section headings need to be in alignment with each other. For example, you can more the credits section further down on the template, and expand the conclusion section. • Please list yourself as the first author. • Remove credentials (e. g. , Ph. D. , MS 3, etc. ) as we are not able to verify and confirm the accuracy in this short time frame. It is fine to identify individuals with institutions – e. g. , Jane Doe, 1 Sam Smith 2 1 -University of Missouri-Kansas City School of Medicine 2 -Truman Medical Center • It is preferred to spell out “University of Missouri-Kansas City School of Medicine. ” • Addresses and dept. names often take up too much space in the header; do not include them. • Do NOT Resize the template. Template is sized accurately for printing. We print your poster for you. • Please include logos for all involved institutions in the header and delete the institutional logos at the bottom of page that are not applicable. If you are not sure of the logo(s) to use, email us at somresearch@umkc. edu



