The Dual Nature of Light In your notes






- Slides: 16

The Dual Nature of Light

In your notes…. . DEFINE LIGHT

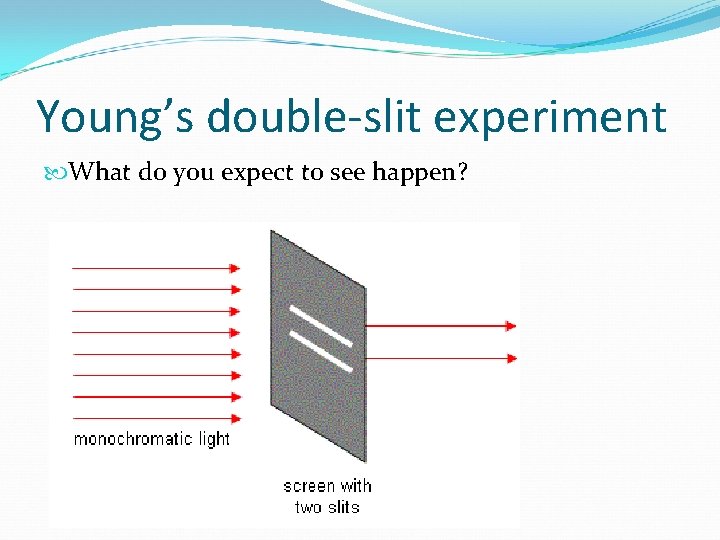
Young’s double-slit experiment What do you expect to see happen?

The Great Debate… Is light a wave or a particle? ? https: //www. youtube. com/watch? v=I uv 6 h. Y 6 zsd 0 Dr. Quantum https: //www. youtube. com/watch? v=Df Pepr. Q 7 o. Gc

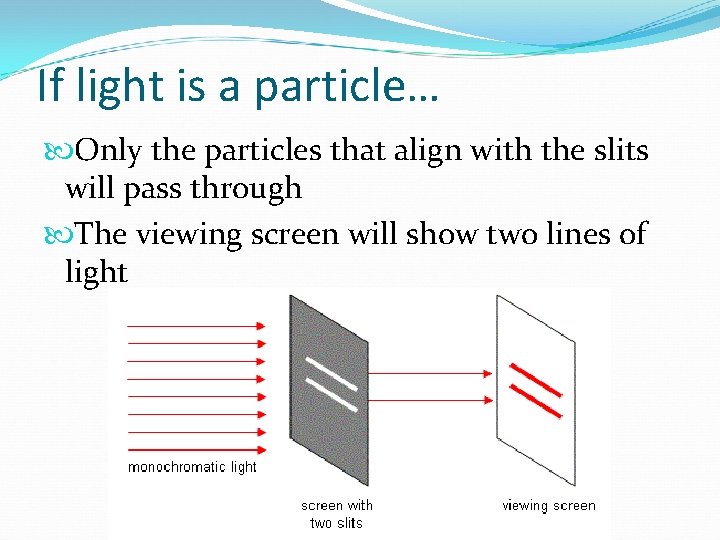
If light is a particle… Only the particles that align with the slits will pass through The viewing screen will show two lines of light

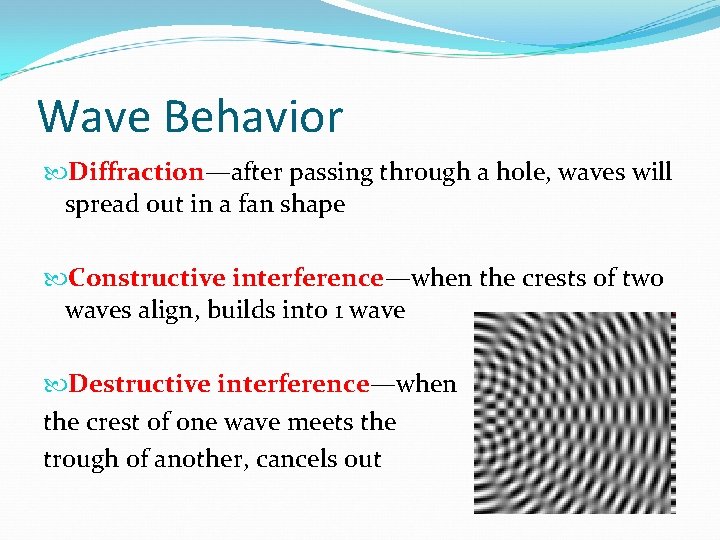
Wave Behavior Diffraction—after passing through a hole, waves will spread out in a fan shape Constructive interference—when the crests of two waves align, builds into 1 wave Destructive interference—when the crest of one wave meets the trough of another, cancels out

If light is a wave… Areas of constructive interference will shine brighter Areas of destructive interference will be black

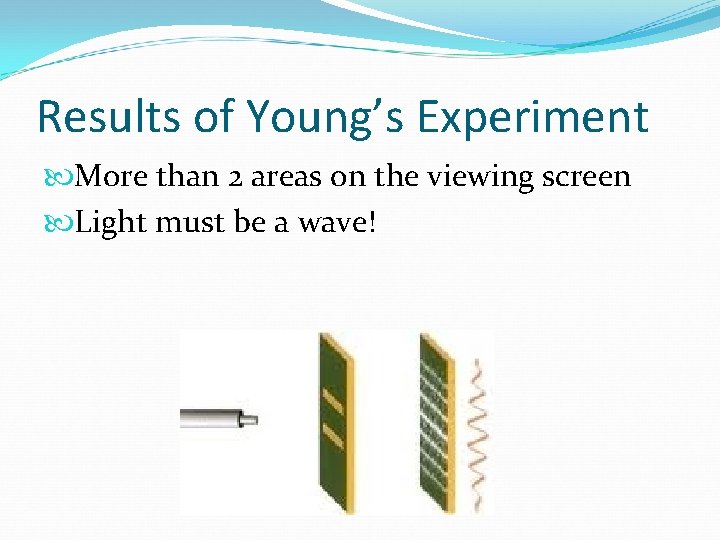
Results of Young’s Experiment More than 2 areas on the viewing screen Light must be a wave!

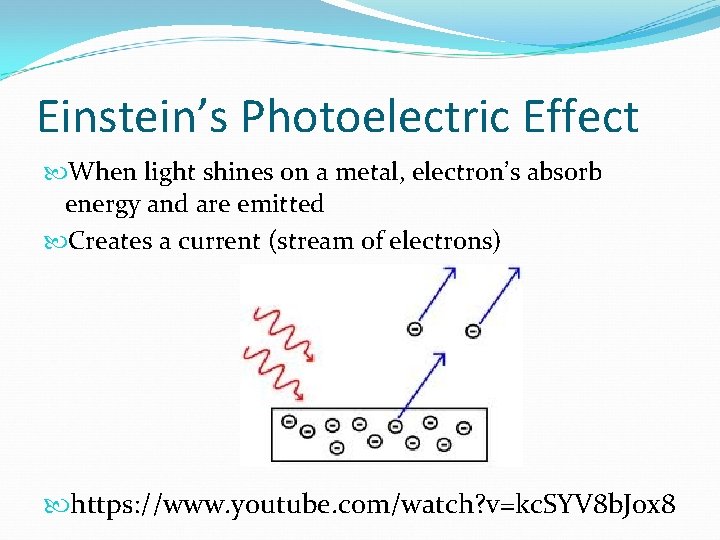
Einstein’s Photoelectric Effect When light shines on a metal, electron’s absorb energy and are emitted Creates a current (stream of electrons) https: //www. youtube. com/watch? v=kc. SYV 8 b. Jox 8

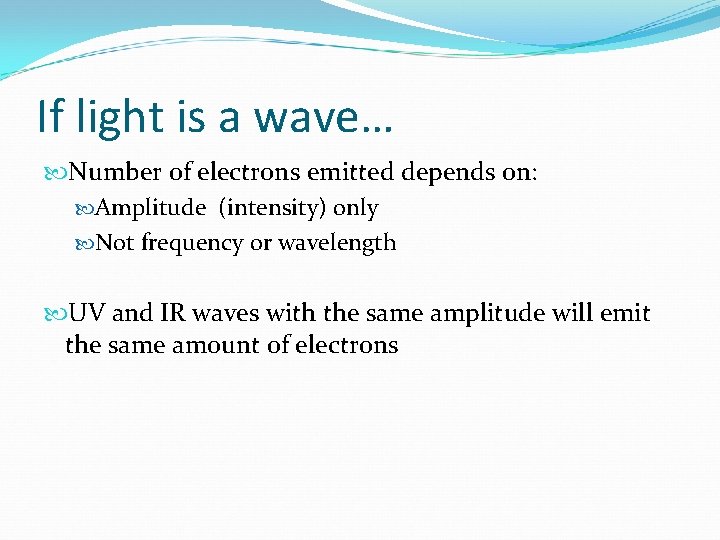
If light is a wave… Number of electrons emitted depends on: Amplitude (intensity) only Not frequency or wavelength UV and IR waves with the same amplitude will emit the same amount of electrons

Photoelectric Experiment Results With constant intensity, higher frequency emits more e Light must be a particle!

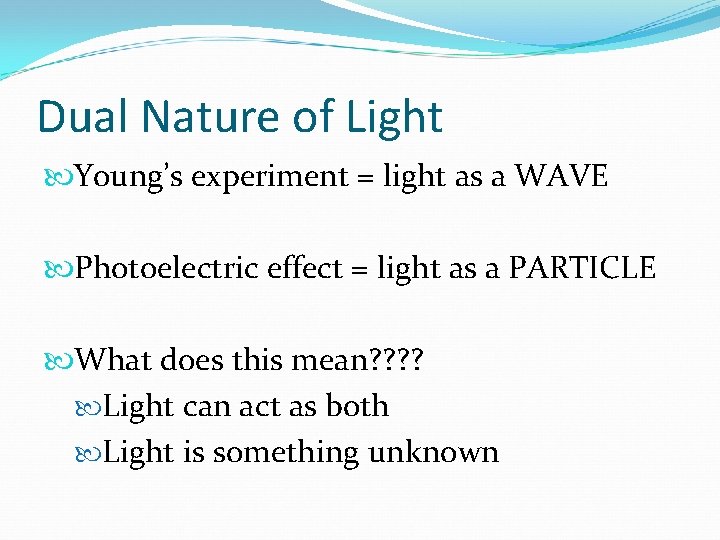
Dual Nature of Light Young’s experiment = light as a WAVE Photoelectric effect = light as a PARTICLE What does this mean? ? Light can act as both Light is something unknown

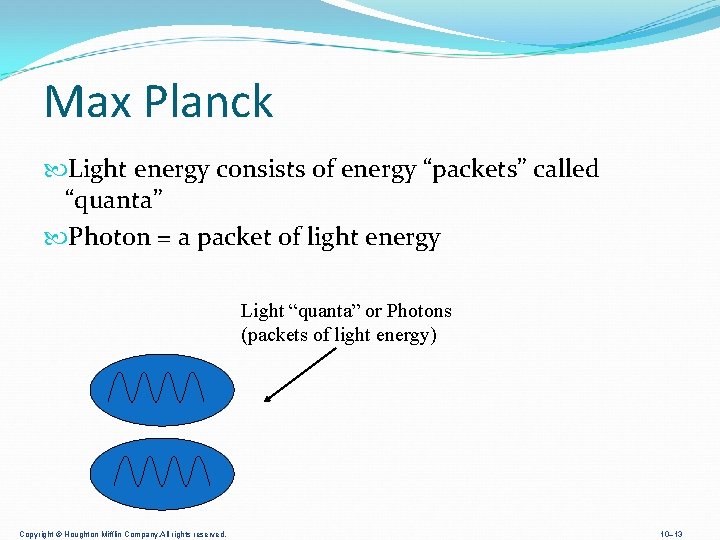
Max Planck Light energy consists of energy “packets” called “quanta” Photon = a packet of light energy Light “quanta” or Photons (packets of light energy) Copyright © Houghton Mifflin Company. All rights reserved. 10– 13

Review Draw the Bohr atom for aluminum. Label each energy level. Example: n=1

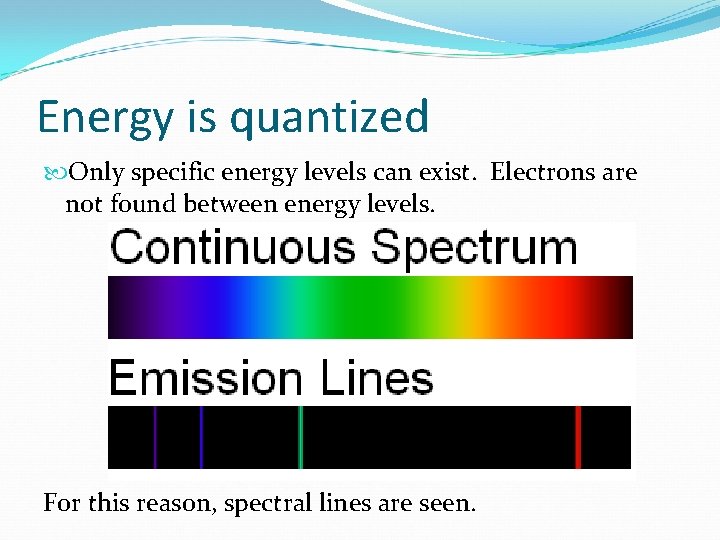
Energy is quantized Only specific energy levels can exist. Electrons are not found between energy levels. For this reason, spectral lines are seen.

Rydberg Equation We can mathematically calculate what wavelength/color that will be emitted by an electron based on what energy levels it transitions to. Let’s calculate!
 Light has a dual nature
Light has a dual nature Energy of light wave
Energy of light wave Uses of concave and convex mirror
Uses of concave and convex mirror Dual nature of light
Dual nature of light Dual nature of light
Dual nature of light Dual nature of light
Dual nature of light Light light light chapter 23
Light light light chapter 23 Into the light chapter 22
Into the light chapter 22 Light light light chapter 22
Light light light chapter 22 Syngenta corn herbicides
Syngenta corn herbicides Give us your hungry your tired your poor
Give us your hungry your tired your poor Nature and nature's laws lay hid in night meaning
Nature and nature's laws lay hid in night meaning Determinace lidské psychiky
Determinace lidské psychiky Nature of science
Nature of science Ib biology nature of science notes
Ib biology nature of science notes Cube wisc
Cube wisc Wave nature
Wave nature